Abstract
Purpose
To investigate the relationship between surgical approach for intraocular tumor biopsy of uveal melanoma and tumor morphologic features such as size and intraocular location and the effect of these variables on diagnostic yield and biopsy outcome.
Methods
Consecutive patients from nine Ocular Oncology centers with uveal melanoma (UM) undergoing tumor biopsy immediately preceding I125 plaque brachytherapy with tissue sent for gene expression profiling (GEP) testing were reviewed retrospectively.
Results
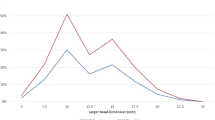
Three hundred sixty patients were included (50% men, mean age 60.2 years). Overall biopsy yield was 99% and 83% for GEP and cytopathology, respectively. Surgeon choice of biopsy approach (trans-vitreal vs. trans-scleral) was found to associate with both tumor location and tumor thickness. A trans-scleral rather than trans-vitreal approach was used more commonly for anteriorly located tumors (92% vs. 38% of posterior tumors, p < 0.001) and thicker tumors (86% vs. 55% of thin tumors, p < 0.001). When performing trans-vitreal biopsies, ocular oncologists with previous vitreoretinal surgery fellowship training were more likely to use wide-field surgical viewing systems, compared with indirect ophthalmoscopy (82.6% vs. 20.6%, p < 0.001). Surgical complications were rare and occurred more frequently with trans-vitreal biopsies (3.6% vs. 0.46%, p = 0.046).
Conclusions
In this multi-center analysis of UM tumor biopsy, surgical yield was high for obtaining tumor tissue for GEP and cytopathology analysis with both trans-scleral and trans-vitreal techniques. Fellowship-trained ocular oncologists’ preferred intraocular biopsy techniques associated strongly with tumor location, tumor thickness, and fellowship training of the surgeon. Short-term complication rates were low.
Similar content being viewed by others
References
Seider MI, Mruthyunjaya P (2018) Molecular prognostics for uveal melanoma. Retina. 38(2):211–219
Coupland SE, Lake SL et al (2013) Molecular pathology of uveal melanoma. Eye (Lond) 27(2):230–242
Onken MD, Worley LA, Char DH et al (2012) Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 119(8):1596–1603
Valsecchi ME, Orloff M, Sato R et al (2018) Adjuvant sunitinib in high-risk patients with uveal melanoma: comparison with institutional controls. Ophthalmology. 125(2):210–217
National Comprehensive Cancer Network. Uveal Melanoma Guidelines (Version 1.2018). https://www.nccn.org/professionals/physician_gls/pdf/uveal.pdf.
Finn AP, Materin MA, Mruthyunjaya P (2018) Choroidal tumor biopsy: a review of the current state and a glance into future techniques. Retina 38(Suppl 1):S79–S87
Berry D, Seider M, Stinnett S et al (2018) Relationship of clinical features and baseline tumor size with gene expression profile status in uveal melanoma: a multi-institutional study. Retina 38(Suppl 1):S79–S87
Mruthyunjaya P, Seider MI, Stinnett S et al (2017) Association between tumor regression rate and gene expression profile after iodine 125 plaque radiotherapy for Uveal melanoma. Ophthalmology. 124(10):1532–1539
Berry DE, Schefler AC, Seider MI et al (2018) Correlation of gene expression profile status and American Joint Commission on Cancer stage in uveal melanoma. Retina. https://doi.org/10.1097/IAE.0000000000002385
Amin MB, Edge S, Greene F et al (2017) AJCC Cancer staging manual, 8th edn. Springer International Publishing, Heidelberg
Castle Biosciences Inc. DecisionDx-UM Summary. http://www.myuvealmelanoma.com/health-care-professionals/decisiondx-um-summary/. Accessed 20 Dec 2018
Kim RS, Chevez-Barrios P, Divatia M et al (2018) Yield, techniques, and complications of transvitreal and transscleral biopsies in small uveal melanoma. JAMA Ophthalmol. 136(5):482–488
Augsburger JJ, Corrêa ZM, Augsburger BD (2015) Frequency and implications of discordant gene expression profile class in posterior uveal melanomas sampled by fine needle aspiration biopsy. Am J Ophthalmol 159(2):248–256
Mashayekhi A, Lim RP, Shields CL et al (2016) Extraocular extension of ciliochoroidal melanoma after transscleral fine-needle aspiration biopsy. Retin Cases Brief Rep. 10(3):289–292
Schefler AC, Gologorsky D, Marr BP et al (2013) Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmol 131(9):1220–1224
Caminal JM, Sanz S, Carreras M et al (2006) Epibulbar seeding at the site of a transvitreal fine-needle aspiration biopsy. Arch Ophthalmol 124(4):587–589
Raja V, Russo A, Coupland S et al (2011) Extraocular seeding of choroidal melanoma after a transretinal biopsy with a 25-gauge vitrector. Retin Cases Brief Rep 5(3):194–196
Singh AD, Turell ME, Topham AK (2011) Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 118(9):1881–1885
Grewal DS, Bhullar PK, Pasricha ND et al (2017) Intraoperative 4-dimensional microscope-integrated optical coherence tomography-guided 27-gauge transvitreal choroidal biopsy for choroidal melanoma. Retina. 37(4):796–799
Grewal DS, Cummings TJ, Mruthyunjaya P (2017) Outcomes of 27-gauge vitrectomy-assisted choroidal and subretinal biopsy. Ophthalmic Surg Lasers Imaging Retina 48(5):406–415
Acknowledgments
The following are Ocular Oncology Study Consortium members:
Duke Eye Center, Durham, NC (coordinating center): Prithvi Mruthyunjaya, MD, MHS (Study PI), Miguel Materin, MD, Michael I. Seider, MD, Duncan E. Berry, MD, Nikolas N. Raufi, MD, Sandra Stinnett, DrPH; University of Miami/Bascom Palmer Eye Institute, Miami, FL: J. William Harbour, MD; University of Southern California/USC Roski Eye Institute, Los Angeles, CA: Jesse L. Berry, MD, and Jonathan Kim, MD; Oregon Health Sciences/Casey Eye Institute, Portland, OR: Alison Skalet, MD, PhD, and Audra Miller, MD; Smilow Cancer Hospital at Yale New Haven, New Haven, CT: Miguel Materin, MD, and Tiffany Liu, MD; University of Michigan, Ann Arbor, MI: Hakan Demirci, MD, and Zeynep G. Ozkurt, MD; Colorado Retina Associates, Rocky Vista University, Denver, CO: Peter Hovland, MD, PhD; Retina Specialists of Michigan, Grand Rapids, MI and Michigan State University, East Lansing, MI: Thomas Aaberg, Jr., MD; Retina Consultants of Houston/Blanton Eye Institute at Houston Methodist Hospital, Houston TX: Amy C. Schefler, MD, Ryan S. Kim, and Anne Tann, MD (Department of Radiation Oncology, Houston Methodist Hospital).
Funding
This study was funded by The Heed Ophthalmic Fellowship - (M.Seider), The Childress Family Foundation (NC), unrestricted departmental funding from Research to Prevent Blindness (New York, NY) and by grant P30 EY010572 from the National Institutes of Health (Bethesda, MD) – (P.Mruthyunjaya).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Prithvi Mruthyunjaya serves as a consultant for Castle Biosciences, Optos Inc., Santen, EyePoint Pharmaceuticals, and Arix Biosciences.
Miguel Materin serves as a consultant for Castle Biosciences.
Amy Schefler serves as a consultant for Castle Biosciences (grant funding), Aura Biosciences (consultant, grant funding); Regeneron (grant funding) and Genentech (consultant, grant funding).
Duncan Berry declares he has no conflict of interest.
Michael I. Seider declares he has no conflict of interest.
Sandra Stinnett declares she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the numerous Institutional Review Boards who reviewed this study (all centers listed below) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not obtained from all individual participants included in the study as this study was retrospective and de-identified.
Disclaimer
The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication were solely made by the authors without influence from the funding organizations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seider, M.I., Berry, D.E., Schefler, A.C. et al. Multi-center analysis of intraocular biopsy technique and outcomes for uveal melanoma: Ocular Oncology Study Consortium report 4. Graefes Arch Clin Exp Ophthalmol 258, 427–435 (2020). https://doi.org/10.1007/s00417-019-04531-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04531-8