Fast Isolation of Flavonoids from the Endemic Species Nolana ramosissima I.M. Johnst and Its Endothelium-Independent Relaxation Effect in Rat Aorta
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of the Compounds in N. ramosissima Methanol and Herbal Tea
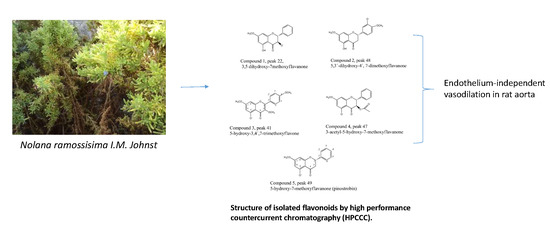
2.1.1. Flavonoids
2.1.2. Fatty Acids
2.1.3. Coumarins
2.1.4. Phenolic Acids
2.2. Fast HPCCC Isolation of Major Compounds in N. ramosissima Methanol Extract
2.3. N. ramosissima Induced Relaxation in Aortic Ring of Rat, Endothelium-Independent Activity
2.4. N. ramosissima Reduced the Contractile Response to KCl and Phenylephrine
2.5. Pure Compounds of N. ramosissima Induced Relaxation
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction
3.4. UHPLC-PDA-MS Instrument
3.5. LC Parameters
3.6. MS Parameters
3.7. Selection of the Solvent System for HPCCC
3.8. HSCCC Separation of N. ramosissima Methanol Extract
3.9. Isolation and Identification of Compounds
3.10. Animals
3.11. Isolation of Aortic Rings
3.12. Vascular Reactivity Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Jewell, C.; Papineau, A.D.; Freyre, R.; Moyle, L.C. Patterns of reproductive isolation in Nolana (Chilean bellflower). Evolution 2012, 66, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Ossa, P.G.; Pérez, F.; Armesto, J.J. Phylogeography of two closely related species of Nolana from the coastal Atacama Desert of Chile: Post-glacial population expansions in response to climate fluctuations. J. Biogeogr. 2013, 40, 2191–2203. [Google Scholar] [CrossRef]
- Chamy, M.C.; Piovano, M.; Garbarino, J.A. Diterpenoids from Nolana elegans. Bol. Soc. Chil. Quim. 2002, 47, 367–370. [Google Scholar] [CrossRef]
- Chamy, M.C.; Garbarino, J.A.; Piovano, E.; Lopez-Perez, J.L.; Nicoletti, M.; Gandolfo, R.; Feliciano, A.S. 9-epi-labdane diterpenoids from Nolana rostrata var. rostrata. Phytochemistry 1997, 45, 797–800. [Google Scholar] [CrossRef]
- Garbarino, J.A.; Chamy, M.C.; Piovano, M.; Gambaro, V. Labdane diterpenoids from Nolana filifolia. Phytochemistry 1988, 27, 1795–1796. [Google Scholar] [CrossRef]
- Garbarino, J.A.; Chamy, M.C.; Montagna, M.P.; Gambaro, V. Sesquiterpenoids in Nolana coelestis. Phytochemistry 1993, 32, 987–989. [Google Scholar] [CrossRef]
- Vio-Michaelis, S.; Apablaza-Hidalgo, G.; Gómez, M.; Peña-Vera, R.; Montenegro, G. Antifungal activity of three Chilean Plant extracts on Botritis Cinerea. Bot. Sci. 2012, 90, 179–183. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Benites, J.; Areche, C.; Sepúlveda, B. Antioxidant capacities and analysis of phenolic compounds in three endemic Nolana species by HPLC-PDA-ESI-MS. Molecules 2015, 20, 11490–11507. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Peng, J.; Yang, G.; Fan, G.; Wu, Y. Preparative isolation and separation of a novel and two known flavonoids from Patrinia villosa Juss by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1092, 235–240. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, M.P.; Lugo-Cervantes, E.; Winterhalter, P.; Jerz, G. Metabolite profiling of polyphenols in peels of Citrus limetta Risso by combination of preparative high-speed countercurrent chromatography and LC-ESI-MS/MS. Food Chem. 2014, 158, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.H.; Si, X.X.; Tong, X.; Li, G.K. Preparation of flavonoids and diarylheptanoid from Alpinia katsumadai hayata by microwave-assisted extraction and high-speed counter-current chromatography. Sep. Purif. Technol. 2011, 81, 265–269. [Google Scholar] [CrossRef]
- Pan, S.B.; Wang, X.; Duan, W.J.; Yu, Z.Y.; Zhang, L.; Liu, W. Preparative isolation and purification of flavonoids from Cuscuta chinensis Lam. by high speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2162–2171. [Google Scholar] [CrossRef]
- Schafer, K.; Winterhalter, P. Application of high speed countercurrent chromatography (HSCCC) to the isolation of kavalactones. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 1703–1716. [Google Scholar] [CrossRef]
- Cheel, J.; Hajek, J.; Kuzma, M.; Saurav, K.; Smykalova, I.; Ondrackova, E.; Urajova, P.; Vu, D.L.; Faure, K.; Kopecky, J.; et al. Application of HPCCC Combined with Polymeric Resins and HPLC for the Separation of Cyclic Lipopeptides Muscotoxins A-C and Their Antimicrobial Activity. Molecules 2018, 23, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Borquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) Fruit: A Source of Bioactive Flavonoid C-Glycosides Isolated by HSCCC and Characterized by HPLC-DAD-ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Di, D.L.; Zheng, Y.Y.; Chen, X.F.; Huang, X.Y.; Feng, S.L. Advance of Application of High Speed Counter-current Chromatography in Separation and Purification of Flavonoids. Chinese J. Anal. Chem. 2011, 39, 269–275. [Google Scholar] [CrossRef]
- Yi, T.; Zhu, L.; Zhu, G.Y.; Tang, Y.N.; Xu, J.; Fan, J.Y.; Zhao, Z.Z.; Chen, H.B. HSCCC-based strategy for preparative separation of in vivo metabolites after administration of an herbal medicine: Saussurea laniceps, a case study. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.; Tang, Y.N.; Zhang, J.Y.; Zhao, Z.Z.; Yang, Z.J.; Chen, H.B. Characterization and determination of six flavonoids in the ethnomedicine “Dragon’s Blood” by UPLC-PAD-MS. Chem. Cent. J. 2012, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.L.; Zhu, L.; Tang, Y.N.; Kwan, H.Y.; Zhao, Z.Z.; Chen, H.B.; Yi, T. Comparative evaluation of chemical profiles of three representative ‘snow lotus’ herbs by UPLC-DAD-QTOF-MS combined with principal component and hierarchical cluster analyses. Drug Test. Anal. 2017, 9, 1105–1115. [Google Scholar] [CrossRef]
- Brito, A.; Areche, C.; Sepulveda, B.; Kennelly, E.J.; Simirgiotis, M.J. Anthocyanin Characterization, Total Phenolic Quantification and Antioxidant Features of Some Chilean Edible Berry Extracts. Molecules 2014, 19, 10936–10955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepulveda, B.; Simirgiotis, M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Borquez, J.; Bartolucci, N.L.; Echiburu-Chau, C.; Winterhalter, P.; Vallejos, J.; Jerz, G.; Simirgiotis, M.J. Isolation of cytotoxic diterpenoids from the Chilean medicinal plant Azorella compacta Phil from the Atacama Desert by high-speed counter-current chromatography. J. Sci. Food Agric. 2016, 96, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, F.; Palacios, J.; R Nwokocha, C.; Bórquez, J.; Simirgiotis, M.J.; Norambuena, I.; Chiong, M.; Paredes, A. Polyphenolic Composition and Hypotensive Effects of Parastrephia quadrangularis (Meyen) Cabrera in Rat. Antioxidants (Basel) 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B.x. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Tattini, M.; Di Ferdinando, M.; Brunetti, C.; Goti, A.; Pollastri, S.; Bellasio, C.; Giordano, C.; Fini, A.; Agati, G. Esculetin and esculin (esculetin 6-O-glucoside) occur as inclusions and are differentially distributed in the vacuole of palisade cells in Fraxinus ornus leaves: A fluorescence microscopy analysis. J. Photochem. Photobiol. B 2014, 140, 28–35. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J. Funct. Foods 2016, 22, 376–388. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Borquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequen. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.L.; Zhao, C.; Shao, Q.J.; Hassan, M. Structural Characterization of Corn Stover Lignin after Hydrogen Peroxide Presoaking Prior to Ammonia Fiber Expansion Pretreatment. Energ. Fuel. 2018, 32, 6022–6030. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, X.L.; Cao, Y.; Shao, Q.J. Application of hydrogen peroxide presoaking prior to ammonia fiber expansion pretreatment of energy crops. Fuel 2017, 205, 184–191. [Google Scholar] [CrossRef]
- Urzua, A.; Modak, B.; Villaroel, L.; Torres, R.; Andrade, L.; Mendoza, L.; Wilkens, M. External flavonoids from Heluitropium megalanthum and H. huascolense (Boraginaceae). Chemotaxonomic considerations. Bol. Soc. Chil. Quím. 2000, 45, 23–29. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon-13 NMR of flavonoids; Elsevier: Michigan, MI, USA, 1989; p. 564. [Google Scholar]
- Torrenegra, R.D.; Rodriguez, O.E. Chemical and biological activity of leaf extracts of Chrola enaleivensis. Nat. Prod. Commun. 2011, 6, 947–950. [Google Scholar]
- Gajhede, M.; Encarnación, R.; Leal, G.C.; Patino, J.C.; Christophersen, C.; Nielsen, P.H. 5-Hydroxy-3,7,4′-trimethoxyflavone. Acta Cryst. 1989, C45, 2012–2014. [Google Scholar] [CrossRef]
- Miri, A.; Monsef-Esfahani, H.R.; Amini, M.; Amanzadeh, Y.g.; Hadjikhoondi, A.; Hajiaghaee, R. Determination of Phenolics and Flavonoid Contens, Antioxidant Capacity and Mayor Flavanoids Structure in Perscicum Boiss. J. An. Vet. Adv. 2011, 10, 1258–1261. [Google Scholar]
- Torres, R.; Modak, B.; Villarroel, L.; Urzua, A.; Delle-Monache, F.; Sanchez-Ferrando, F. Flavonoides del exudado resinoso de Heliotropium sinuatum. Bol. Soc. Chil. Quím. 1996, 41, 195–197. [Google Scholar]
- Smolarz, H.D.; Mendyk, E.; Bogucka-Kocka, A. Pinostrobin-An anti-Leukemic Flavonoid from Polygonum lapanthifolium L. ssp. Nodosum (Pers.) Dans. Zeitsch. Naturforsch. 2006, 61c, 64–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, I.; Simirgiotis, M.J.; Brito, A.; Werner, M.R.; Bórquez, J.; Winterhalter, P.; Cárdenas, A. A non-centrosymmetric polymorph of 5-hydroxy-7-methoxy-2-phenylchroman-4-one. J. Chil. Chem. Soc. 2015, 60, 2864–2866. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, R.M.; Schwartz, K.; Murad, F. Effects of Na+, K+-pump inhibitors and membrane depolarizing agents on acetylcholine-induced endothelium-dependent relaxation and cyclic GMP accumulation in rat aorta. Eur. J. Pharmacol. 1985, 110, 203–209. [Google Scholar] [CrossRef]
- Vesely, D.L. Ergotamine and dihydroergotamine enhance guanylate cyclase activity. Res. Comm. Chem. Pathol. Pharmacol. 1983, 40, 245–254. [Google Scholar]
- Da Silva, F.H.; Claudino, M.A.; Báu, F.R.; Rojas-Moscoso, J.A.; Mónica, F.Z.; De Nucci, G.; Antunes, E. Vas deferens smooth muscle responses to the nitric oxide-independent soluble guanylate cyclase stimulator BAY 41-2272. Eur. J. Pharmacol. 2012, 688, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.R.; Marletta, M.A. Structure and regulation of soluble guanylate cyclase. Annu. Rev. Biochem. 2012, 81, 533–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lies, B.; Groneberg, D.; Gambaryan, S.; Friebe, A. Lack of effect of ODQ does not exclude cGMP signalling via NO-sensitive guanylyl cyclase. Br. J. Pharmacol. 2013, 170, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bórquez, J.; Kennelly, E.J.; Simirgiotis, M.J. Activity guided isolation of isoflavones and hyphenated HPLC-PDA-ESI-ToF-MS metabolome profiling of Azorella madreporica Clos. from northern Chile. Food Res. Int. 2013, 52, 288–297. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Elution−Extrusion Countercurrent Chromatography. Use of the Liquid Nature of the Stationary Phase To Extend the Hydrophobicity Window. Anal. Chem. 2003, 75, 5886–5894. [Google Scholar] [CrossRef]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladia, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Drugs | Log (IC50) (g/mL) |
|---|---|
| Control | 2.38 ± 0.12 |
| Endo-denuded | 2.37 ± 0.06 |
| L-NAME | 2.35 ± 0.10 |
| Methylene blue | |
| ODQ | 3.51 ± 0.38 * |
| Drugs | Log (EC50) |
|---|---|
| KCl (mM) | |
| Control | 1.57 ± 0.15 |
| Nr | 1.63 ± 0.50 |
| Nimodipine | |
| PE (nM) Control | −7.47 ± 0.10 |
| Nr | −7.18 ± 0.26 |
| Nimodipine | −7.05 ± 0.14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cifuentes, F.; Palacios, J.; Bórquez, J.; Paredes, A.; Parra, C.; Bravo, A.; Simirgiotis, M.J. Fast Isolation of Flavonoids from the Endemic Species Nolana ramosissima I.M. Johnst and Its Endothelium-Independent Relaxation Effect in Rat Aorta. Molecules 2020, 25, 520. https://doi.org/10.3390/molecules25030520
Cifuentes F, Palacios J, Bórquez J, Paredes A, Parra C, Bravo A, Simirgiotis MJ. Fast Isolation of Flavonoids from the Endemic Species Nolana ramosissima I.M. Johnst and Its Endothelium-Independent Relaxation Effect in Rat Aorta. Molecules. 2020; 25(3):520. https://doi.org/10.3390/molecules25030520
Chicago/Turabian StyleCifuentes, Fredi, Javier Palacios, Jorge Bórquez, Adrián Paredes, Claudio Parra, Alejandra Bravo, and Mario J. Simirgiotis. 2020. "Fast Isolation of Flavonoids from the Endemic Species Nolana ramosissima I.M. Johnst and Its Endothelium-Independent Relaxation Effect in Rat Aorta" Molecules 25, no. 3: 520. https://doi.org/10.3390/molecules25030520