Abstract
Optimal care for familial hypercholesterolaemia (FH) requires patient-centred management, multidisciplinary teamwork, involvement of primary care practitioners, patient networks, support groups and high-quality clinical registries, implemented through models of care adapted to FH. Models of care — evidence-based and context-specific frameworks that aim to deliver the highest quality of care for patients and their families — allow the application of precision and multidisciplinary medicine to FH care and can serve as paradigms for the prevention of premature atherosclerotic cardiovascular disease in all at-risk patients and families worldwide. The exponential growth in the number of publications on diverse aspects of FH has provided new knowledge for developing essential elements of existing models of care. These elements include clinical diagnostic criteria and genetic testing; risk restratification strategies; LDL-cholesterol treatment targets; management protocols for children; care of women in pregnancy; use of pharmacotherapies, including ezetimibe and PCSK9 inhibitors; use of lipoprotein apheresis for severe FH; and addressing barriers to care. However, substantial gaps remain that need to be addressed by a broad research agenda, implementation strategies and global collaboration and advocacy, aimed at improving the uptake, cost-effectiveness and routine implementation of evidence-based standards. In this Review, we summarize the dramatic increase in knowledge that informs adaptive models of care, with an emphasis on articles published since 2014.
Key points
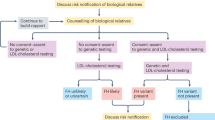
Effective identification of familial hypercholesterolaemia (FH) requires the coordination of several screening strategies, with an emphasis on early detection and a central role for primary care.
Clinical diagnostic criteria for FH can be imprecise and need refining with affordable genetic testing; detection of a pathogenic mutation has prognostic utility and allows cascade testing and the initiation of treatment in childhood.
Incident atherosclerotic cardiovascular disease is variable in FH and can be predicted by genetic and phenotypic factors, including LDL-cholesterol burden and non-invasive imaging methods.
Management entails the timely lowering of LDL-cholesterol burden by lifestyle modifications and the use of statins, followed by ezetimibe and PCSK9 inhibitors if required; women and children need special care, and lipoprotein apheresis is indicated in patients with severe FH.
Effective models of care require multidisciplinary teams, patient networks, registries and research programmes; implementation remains a major challenge.
The evidence reviewed can be used to design adaptive models of care for FH.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Khera, A. V. & Kathiresan, S. Genetics of coronary artery disease: discovery, biology and clinical translation. Nat. Rev. Genet. 18, 331–344 (2017).
Garg, A., Garg, V., Hegele, R. A. & Lewis, G. F. Practical definitions of severe versus familial hypercholesterolaemia and hypertriglyceridaemia for adult clinical practice. Lancet Diabetes Endocrinol. 7, 880–886 (2019).
Watts, G. F. et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int. J. Cardiol. 171, 309–325 (2014).
Berberich, A. J. & Hegele, R. A. The complex molecular genetics of familial hypercholesterolaemia. Nat. Rev. Cardiol. 16, 9–20 (2018).
Sturm, A. C. et al. Clinical genetic testing for familial hypercholesterolemia: JACC scientific expert panel. J. Am. Coll. Cardiol. 72, 662–680 (2018).
Nordestgaard, B. et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart J. 34, 3478–3490 (2013).
Gidding, S. S. et al. The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation 132, 2167–2192 (2015).
Wiegman, A. et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur. Heart J. 36, 2425–2437 (2015).
Vallejo-Vaz, A. J. et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries - the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 277, 234–255 (2018).
Representatives of the Global Familial Hypercholesterolemia Community. Reducing the clinical and public health burden of familial hypercholesterolemia — a global call to action. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2019.5173 (2020).
Ray, K. K. & Watts, G. F. Improving the global care of familial hypercholesterolaemia: starting the ball rolling. Atherosclerosis 277, 230–233 (2018).
Ray, K. K. & Hovingh, G. K. Familial hypercholesterolaemia: a common disease. Eur. Heart J. 37, 1395–1397 (2016).
Defesche, J. C. et al. Familial hypercholesterolaemia. Nat. Rev. Dis. Primers 3, 17093 (2017).
Watts, G. F. et al. Familial hypercholesterolaemia: a model of care for Australasia. Atheroscler. Suppl. 12, 221–263 (2011).
Public Health England. Familial hypercholesterolaemia: implementing a systems approach to detection and management. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/731873/familial_hypercholesterolaemia_implementation_guide.pdf (2018).
Akioyamen, L. E. et al. Estimating the prevalence of heterozygous familial hypercholesterolaemia: a systematic review and meta-analysis. BMJ Open 7, e016461 (2017).
Umans-Eckenhausen, M. A., Defesche, J. C., Sijbrands, E. J., Scheerder, R. L. & Kastelein, J. J. Review of first 5 years of screening for familial hypercholesterolaemia in the Netherlands. Lancet 357, 165–168 (2001).
Leren, T. P. et al. Application of molecular genetics for diagnosing familial hypercholesterolemia in Norway: results from a family-based screening program. Semin. Vasc. Med. 4, 75–85 (2004).
Alver, M. et al. Recall by genotype and cascade screening for familial hypercholesterolemia in a population-based biobank from Estonia. Genet. Med. 21, 1173–1180 (2019).
Jackson, C. L. et al. Identifying familial hypercholesterolemia using a blood donor screening program with more than 1 million volunteer donors. JAMA Cardiol. 4, 685–689 (2019).
Nanchen, D. et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur. Heart J. 36, 2438–2445 (2015).
Kreissl, A., Walleczek, N., Espina, P. R., Hallwirth, U. & Greber-Platzer, S. Selective screening for familial hypercholesterolemia in Austrian children - first year results. BMC Pediatr. 19, 208 (2019).
Kerr, M. et al. Cost effectiveness of cascade testing for familial hypercholesterolaemia, based on data from familial hypercholesterolaemia services in the UK. Eur. Heart J. 38, 1832–1839 (2017).
Ademi, Z. et al. Cascade screening based on genetic testing is cost-effective: evidence for the implementation of models of care for familial hypercholesterolaemia. J. Clin. Lipidol. 8, 390–400 (2014).
Louter, L., Defesche, J. & Roeters van Lennep, J. Cascade screening for familial hypercholesterolemia: practical consequences. Atheroscler. Suppl. 30, 77–85 (2017).
Brett, T., Qureshi, N., Gidding, S. & Watts, G. F. Screening for familial hypercholesterolaemia in primary care: time for general practice to play its part. Atherosclerosis 277, 399–406 (2018).
Safarova, M. S., Liu, H. & Kullo, I. J. Rapid identification of familial hypercholesterolemia from electronic health records: the SEARCH study. J. Clin. Lipidol. 10, 1230–1239 (2016).
Banda, J. M. et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit. Med. 2, 23 (2019).
Myers, K. D. et al. Precision screening for familial hypercholesterolaemia: a machine learning study applied to electronic health encounter data. Lancet Digit. Health 1, e393–e402 (2019).
Pears, R. et al. The reduced cost of providing a nationally recognised service for familial hypercholesterolaemia. Open Heart 1, e000015 (2014).
Morris, J. K., Wald, D. S. & Wald, N. J. The evaluation of cascade testing for familial hypercholesterolemia. Am. J. Med. Genet. A 158, 78–84 (2012).
Wald, D. S. et al. Child–parent familial hypercholesterolemia screening in primary care. N. Engl. J. Med. 375, 1628–1637 (2016).
Bowman, F. L. et al. Identifying perceptions and preferences of the general public concerning universal screening of children for familial hypercholesterolaemia. Public Health Genomics 22, 23–35 (2019).
McKay, A. J. et al. Universal screening at age 1–2 years as an adjunct to cascade testing for familial hypercholesterolaemia in the UK: a cost-utility analysis. Atherosclerosis 275, 434–443 (2018).
Klančar, G. et al. Universal screening for familial hypercholesterolemia in children. J. Am. Coll. Cardiol. 66, 1250–1257 (2015).
Georges, N. et al. Universal screening program for lipid disorders in two to ten years old Lebanese children: a new approach. Int. J. Pediatr. Adolesc. Med. 6, 101–108 (2019).
de Ferranti, S. D. et al. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J. Pediatr. 185, 99–105.e2 (2017).
Mach, F. et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 41, 111–188 (2019).
Chan, D. C. et al. A comparative analysis of phenotypic predictors of mutations in familial hypercholesterolemia. J. Clin. Endocrinol. Metab. 103, 1704–1714 (2018).
Haralambos, K., Ashfield-Watt, P. & McDowell, I. F. Diagnostic scoring for familial hypercholesterolaemia in practice. Curr. Opin. Lipidol. 27, 367–374 (2016).
Haralambos, K. et al. Clinical experience of scoring criteria for familial hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis 240, 190–196 (2015).
Harada-Shiba, M. et al. Guidelines for diagnosis and treatment of familial hypercholesterolemia 2017. J. Atheroscler. Thromb. 25, 751–770 (2018).
Atherosclerosis and Coronary Heart Disease Group of the Chinese Society of Cardiology of Chinese Medical Association and Editorial Board of Chinese Journal of Cardiology. Chinese expert consensus on screening, diagnosis and treatment of familial hypercholesterolemia. Zhonghua Xin Xue Guan Bing Za Zhi 46, 99–103 (2018).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 73, e285–e350 (2019).
Bell, D. A. et al. Familial hypercholesterolaemia in primary care: knowledge and practices among general practitioners in Western Australia. Heart Lung Circ. 23, 309–313 (2014).
Withycombe, B., Winden, J. C., Hassanyn, R., Duell, P. B. & Ito, M. K. The extent of familial hypercholesterolemia instruction in US schools and colleges of medicine, pharmacy, and osteopathic medicine. J. Clin. Lipidol. 9, 281–288 (2015).
Azraii, A. B. et al. Knowledge, awareness and practice regarding familial hypercholesterolaemia among primary care physicians in Malaysia: the importance of professional training. Atherosclerosis 277, 508–516 (2018).
Langsted, A., Kamstrup, P. R., Benn, M., Tybjærg-Hansen, A. & Nordestgaard, B. G. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol. 4, 577–587 (2016).
Chan, D. C. et al. Effect of lipoprotein(a) on the diagnosis of familial hypercholesterolemia: does it make a difference in the clinic? Clin. Chem. 65, 1258–1266 (2019).
Harada-Shiba, M. et al. Guidance for pediatric familial hypercholesterolemia 2017. J. Atheroscler. Thromb. 25, 539–553 (2018).
Starr, B. et al. Development of sensitive and specific age-and gender-specific low-density lipoprotein cholesterol cutoffs for diagnosis of first-degree relatives with familial hypercholesterolaemia in cascade testing. Clin. Chem. Lab. Med. 46, 791–803 (2008).
Pérez de Isla, L. et al. Coronary heart disease, peripheral arterial disease, and stroke in familial hypercholesterolaemia. Arterioscler. Thromb. Vasc. Biol. 36, 2004–2010 (2016).
Besseling, J. et al. Selection of individuals for genetic testing for familial hypercholesterolaemia: development and external validation of a prediction model for the presence of a mutation causing familial hypercholesterolaemia. Eur. Heart J. 38, 565–573 (2016).
Santos, R. D. et al. Defining severe familial hypercholesterolaemia and the implications for clinical management: a consensus statement from the International Atherosclerosis Society Severe Familial Hypercholesterolemia Panel. Lancet Diabetes Endocrinol. 4, 850–861 (2016).
Humphries, S. E. et al. Coronary heart disease mortality in severe vs. non-severe familial hypercholesterolaemia in the Simon Broome Register. Atherosclerosis 281, 207–212 (2019).
Hooper, A. J., Burnett, J. R., Bell, D. A. & Watts, G. F. The present and the future of genetic testing in familial hypercholesterolemia: opportunities and caveats. Curr. Atheroscler. Rep. 20, 31 (2018).
Larsen, L. E., Stoekenbroek, R. M., Kastelein, J. J. & Holleboom, A. G. Moving targets: recent advances in lipid-lowering therapies. Arterioscler. Thromb. Vasc. Biol. 39, 349–359 (2019).
Khera, A. V. et al. Diagnostic yield and clinical utility of sequencing familial hypercholesterolemia genes in patients with severe hypercholesterolemia. J. Am. Coll. Cardiol. 67, 2578–2589 (2016).
Abul-Husn, N. S. et al. Genetic identification of familial hypercholesterolemia within a single U.S. health care system. Science 354, aaf7000 (2016).
Benn, M., Watts, G. F., Tybjærg-Hansen, A. & Nordestgaard, B. G. Mutations causative of familial hypercholesterolaemia: screening of 98 098 individuals from the Copenhagen General Population Study estimated a prevalence of 1 in 217. Eur. Heart J. 37, 1384–1394 (2016).
Amor-Salamanca, A. et al. Genetically confirmed familial hypercholesterolemia in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 70, 1732–1740 (2017).
Ference, B. A. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38, 2459–2472 (2017).
Sharifi, M. et al. Greater preclinical atherosclerosis in treated monogenic familial hypercholesterolemia vs. polygenic hypercholesterolemia. Atherosclerosis 263, 405–411 (2017).
Trinder, M. et al. Risk of premature atherosclerotic disease in patients with monogenic versus polygenic familial hypercholesterolemia. J. Am. Coll. Cardiol. 74, 512–522 (2019).
Roberts, M. C. et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff. 37, 801–808 (2018).
George, R., Kovak, K. & Cox, S. L. Aligning policy to promote cascade genetic screening for prevention and early diagnosis of heritable diseases. J. Genet. Couns. 24, 388–399 (2015).
Knowles, J. W., Rader, D. J. & Khoury, M. J. Cascade screening for familial hypercholesterolemia and the use of genetic testing. J. Am. Med. Assoc. 318, 381–382 (2017).
National Institute for Health and Care Excellence. Familial hypercholesterolaemia: identification management. https://www.nice.org.uk/guidance/cg71 (2019).
Brunham, L. R. et al. Canadian Cardiovascular Society position statement on familial hypercholesterolemia: update 2018. Can. J. Cardiol. 34, 1553–1563 (2018).
Hagger, M. S. et al. Health literacy in familial hypercholesterolemia: a cross-national study. Eur. J. Prev. Cardiol. 25, 936–943 (2018).
Ormond, K. E. et al. Developing a conceptual, reproducible, rubric-based approach to consent and result disclosure for genetic testing by clinicians with minimal genetics background. Genet. Med. 21, 727 (2019).
Sarraju, A. & Knowles, J. W. Genetic testing and risk scores: impact on familial hypercholesterolemia. Front. Cardiovasc. Med. 6, 5 (2019).
Raal, F. J. et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. Lancet Diabetes Endocrinol. 5, 280–290 (2017).
Cuchel, M. et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. a position paper from the consensus panel on familial hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 35, 2146–2157 (2014).
Futema, M., Bourbon, M., Williams, M. & Humphries, S. E. Clinical utility of the polygenic LDL-C SNP score in familial hypercholesterolemia. Atherosclerosis 277, 457–463 (2018).
Nikkola, E. et al. Family-specific aggregation of lipid GWAS variants confers the susceptibility to familial hypercholesterolemia in a large Austrian family. Atherosclerosis 264, 58–66 (2017).
Iacocca, M. A. et al. ClinVar database of global familial hypercholesterolemia-associated DNA variants. Hum. Mutat. 39, 1631–1640 (2018).
Safarova, M. S. et al. Variability in assigning pathogenicity to incidental findings: insights from LDLR sequence linked to the electronic health record in 1013 individuals. Eur. J. Hum. Genet. 25, 410 (2017).
Hendricks-Sturrup, R. M., Mazor, K. M., Sturm, A. C. & Lu, C. Y. Barriers and facilitators to genetic testing for familial hypercholesterolemia in the United States: a review. J. Pers. Med. 9, 32 (2019).
Mata, P., Alonso, R. & de Isla, L. P. Atherosclerotic cardiovascular disease risk assessment in familial hypercholesterolemia: does one size fit all? Curr. Opin. Lipidol. 29, 445–452 (2018).
Paquette, M. & Baass, A. Predicting cardiovascular disease in familial hypercholesterolemia. Curr. Opin. Lipidol. 29, 299–306 (2018).
Alonso, R. et al. Lipoprotein(a) levels in familial hypercholesterolaemia: an important predictor for cardiovascular disease independent of the type of LDL-receptor mutation. J. Am. Coll. Cardiol. 63, 1982–1989 (2014).
Chan, D. C. et al. Elevated lipoprotein(a), hypertension and renal insufficiency as predictors of coronary artery disease in patients with genetically confirmed heterozygous familial hypercholesterolemia. Int. J. Cardiol. 201, 633–638 (2015).
Paré, G. et al. Lipoprotein(a) Levels and the risk of myocardial infarction among 7 ethnic groups. Circulation 139, 1472–1482 (2019).
Vuorio, A., Watts, G. F. & Kovanen, P. T. Lipoprotein(a) as a risk factor for calcific aortic valvulopathy in heterozygous familial hypercholesterolemia. Atherosclerosis 281, 25–30 (2019).
Mundal, L. J. et al. Association of low-density lipoprotein cholesterol with risk of aortic valve stenosis in familial hypercholesterolemia. JAMA Cardiol. 4, 1156–1159 (2019).
Mangili, L. C. et al. Epicardial fat is associated with severity of subclinical coronary atherosclerosis in familial hypercholesterolemia. Atherosclerosis 254, 73–77 (2016).
Tada, H. et al. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur. Heart J. 38, 1573–1579 (2017).
Emanuelsson, F., Nordestgaard, B. G. & Benn, M. Familial hypercholesterolemia and risk of peripheral arterial disease and chronic kidney disease. J. Clin. Endocrinol. Metab. 103, 4491–4500 (2018).
Pérez de Isla, L. et al. Predicting cardiovascular events in familial hypercholesterolemia: The SAFEHEART registry. Circulation 135, 2133–2144 (2017).
Paquette, M. et al. Cardiovascular disease in familial hypercholesterolemia: validation and refinement of the montreal-FH-SCORE. J. Clin. Lipidol. 11, 1161–1167.e3 (2017).
Wilson, D. P. et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J. Clin. Lipidol. 13, 374–392 (2019).
Paquette, M. et al. The 9p21.3 locus and cardiovascular risk in familial hypercholesterolemia. J. Clin. Lipidol. 11, 406–412 (2017).
Paquette, M., Dufour, R. & Baass, A. ABO blood group is a cardiovascular risk factor in patients with familial hypercholesterolemia. J. Clin. Lipidol. 12, 383–389.e1 (2018).
Paquette, M. et al. Polygenic risk score predicts prevalence of cardiovascular disease in patients with familial hypercholesterolemia. J. Clin. Lipidol. 11, 725–732.e5 (2017).
Khera, A. V. et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 10, 1219–1224 (2018).
Zhao, P. J. et al. Genetic determinants of myocardial infarction risk in familial hypercholesterolemia. CJC Open. 1, 225–230 (2019).
Ellis, K. L. et al. Value of measuring lipoprotein(a) during cascade testing for familial hypercholesterolemia. J. Am. Coll. Cardiol. 73, 1029–1039 (2019).
Vuorio, A., Watts, G. F., Schneider, W. J., Tsimikas, S. & Kovanen, P. T. Familial hypercholesterolemia and elevated lipoprotein(a): double heritable risk and new therapeutic opportunities. J. Intern. Med. 287, 2–18 (2020).
Ohta, N. et al. Proprotein convertase subtilisin/kexin 9 V4I variant with LDLR mutations modifies the phenotype of familial hypercholesterolemia. J. Clin. Lipidol. 10, 547–555.e5 (2016).
Cegla, J. et al. HEART UK consensus statement on lipoprotein(a) - a call to action. Atherosclerosis 291, 62–70 (2019).
Sharifi, M., Rakhit, R. D., Humphries, S. E. & Nair, D. Cardiovascular risk stratification in familial hypercholesterolaemia. Heart 102, 1003–1008 (2016).
Miname, M. H. et al. Coronary artery calcium and cardiovascular events in patients with familial hypercholesterolemia receiving standard lipid-lowering therapy. J. Am. Coll. Cardiol. Cardiovasc. Imaging 12, 1797–1804 (2019).
Knuuti, J. et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2019).
Kusters, D. M., Wiegman, A., Kastelein, J. J. & Hutten, B. A. Carotid intima-media thickness in children with familial hypercholesterolemia. Circ. Res. 114, 307–310 (2013).
Braamskamp, M. J. et al. Effect of rosuvastatin on carotid intima-media thickness in children with heterozygous familial hypercholesterolemia: the CHARON study. Circulation 136, 359–366 (2017).
Luirink, I. et al. 20-year follow-up of statins in children with familial hypercholesterolaemia. N. Engl. J. Med. 381, 1547–1556 (2019).
Tada, H. et al. Assessments of carotid artery plaque burden in patients with familial hypercholesterolemia. Am. J. Cardiol. 120, 1955–1960 (2017).
Spence, J. D. Approaching automated 3-dimensional measurement of atherosclerotic plaque volume. J. Am. Coll. Cardiol. 70, 314–317 (2017).
López-Melgar, B. et al. Subclinical atherosclerosis burden by 3D ultrasound in mid-life: the PESA Study. J. Am. Coll. Cardiol. 70, 301–313 (2017).
Doris, M. K., Dweck, M. R. & Fayad, Z. A. The future of imaging in cardiovascular disease intervention trials: 2017 and beyond. Curr. Opin. Lipidol. 27, 605–614 (2016).
Gupta, A. et al. The identification of calcified coronary plaque is associated with initiation and continuation of pharmacological and lifestyle preventive therapies: a systematic review and meta-analysis. J. Am. Coll. Cardiol. Cardiovasc. Imaging 10, 833–842 (2017).
Shapiro, M. D. & Blankstein, R. Reclassifying risk in familial hypercholesterolemia: the power of a coronary artery calcium score of zero. J. Am. Coll. Cardiol. Cardiovasc. Imaging 12, 1805–1807 (2018).
Puri, R. et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 65, 1273–1282 (2015).
Chiva-Blanch, G. et al. Liquid biopsy of extracellular microvesicles maps coronary calcification and atherosclerotic plaque in asymptomatic patients with familial hypercholesterolemia: a computed tomographic angiography imaging study. Arterioscler. Thromb. Vasc. Biol. 39, 945–955 (2019).
Pérez de Isla, L. et al. Coronary computed tomographic angiography findings and their therapeutic implications in asymptomatic patients with familial hypercholesterolemia: lessons from the SAFEHEART study. J. Clin. Lipidol. 12, 948–957 (2018).
Tada, H. et al. Assessment of coronary atherosclerosis in patients with familial hypercholesterolemia by coronary computed tomography angiography. Am. J. Cardiol. 115, 724–729 (2015).
Tada, H. et al. Assessment of arterial stiffness in patients with familial hypercholesterolemia. J. Clin. Lipidol. 12, 397–402.e2 (2018).
Beheshti, S., Madsen, C. M., Varbo, A., Benn, M. & Nordestgaard, B. G. Relationship of familial hypercholesterolemia and high LDL cholesterol to ischemic stroke: the Copenhagen general population study. Circulation 138, 578–589 (2018).
van Wijk, D. F. et al. Nonpharmacological lipoprotein apheresis reduces arterial inflammation in familial hypercholesterolemia. J. Am. Coll. Cardiol. 64, 1418–1426 (2014).
Bos, S. et al. Novel protein biomarkers associated with coronary artery disease in statin-treated patients with familial hypercholesterolemia. J. Clin. Lipidol. 11, 682–693 (2017).
Besseling, J., Hovingh, G. K., Huijgen, R., Kastelein, J. J. P. & Hutten, B. A. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J. Am. Coll. Cardiol. 68, 252–260 (2016).
Vallejo-Vaz, A. J. et al. Low-density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of low-density lipoprotein cholesterol levels of 190 mg/dL or above: analyses from the WOSCOPS (West of Scotland Coronary Prevention Study) 5-year randomized trial and 20-year observational follow-up. Circulation 136, 1878–1891 (2017).
Ridker, P. M. et al. Cardiovascular event reduction with PCSK9 inhibition among 1578 patients with familial hypercholesterolemia: results from the SPIRE randomized trials of bococizumab. J. Clin. Lipidol. 12, 958–965 (2018).
Barrett, B., Ricco, J., Wallace, M., Kiefer, D. & Rakel, D. Communicating statin evidence to support shared decision-making. BMC Fam. Pract. 17, 41 (2016).
Sepucha, K. R. & Scholl, I. Measuring shared decision making: a review of constructs, measures, and opportunities for cardiovascular care. Circ. Cardiovasc. Qual. Outcomes 7, 620–626 (2014).
Kinnear, F. J. et al. Enablers and barriers to treatment adherence in heterozygous familial hypercholesterolaemia: a qualitative evidence synthesis. Br. Med. J. Open. 9, e030290 (2019).
Spatz, E. S. & Spertus, J. A. Shared decision making: a path toward improved patient-centered outcomes. Circ. Cardiovasc. Qual. Outcomes 5, e75–e77 (2012).
Hagger, M. S. et al. Effects of medication, treatment, and behavioral beliefs on intentions to take medication in patients with familial hypercholesterolemia. Atherosclerosis 277, 493–501 (2018).
Robinson, J. G. et al. Enhancing the value of PCSK9 monoclonal antibodies by identifying patients most likely to benefit. J. Clin. Lipidol. 13, 525–537 (2019).
Jellinger, P. S. et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr. Pract. 23, 1–87 (2017).
Jacobson, T. A. et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 1. J. Clin. Lipidol. 9, 129–169 (2015).
National Institute for Health and Clinical Excellence. Alirocumab treating primary hypercholesterolaemia and mixed dyslipidaemia. https://www.nice.org.uk/guidance/ta393/resources/alirocumab-for-treatingprimary-hypercholesterolaemia-and-mixeddyslipidaemia-pdf-82602908493253 (2016).
National Institute for Health and Clinical Excellence. Evolocumab treating primary hypercholesterolaemia mixed dyslipidaemia. https://www.nice.org.uk/guidance/ta394/resources/evolocumab-for-treatingprimary-hypercholesterolaemia-and-mixeddyslipidaemia-pdf-82602910172869 (2016).
Pijlman, A. H. et al. Evaluation of cholesterol lowering treatment of patients with familial hypercholesterolemia: a large cross-sectional study in the Netherlands. Atherosclerosis 209, 189–194 (2010).
Perez de Isla, L. et al. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J. Am. Coll. Cardiol. 67, 1278–1285 (2016).
Bogsrud, M. P. et al. LDL-cholesterol goal achievement, cardiovascular disease, and attributed risk of Lp(a) in a large cohort of predominantly genetically verified familial hypercholesterolemia. J. Clin. Lipidol. 13, 279–286 (2019).
Galema-Boers, A. M., Lenzen, M. J., Engelkes, S. R., Sijbrands, E. J. & van Lennep, J. E. R. Cardiovascular risk in patients with familial hypercholesterolemia using optimal lipid-lowering therapy. J. Clin. Lipidol. 12, 409–416 (2018).
Annemans, L., Packard, C. J., Briggs, A. & Ray, K. K. ‘Highest risk–highest benefit’strategy: a pragmatic, cost-effective approach to targeting use of PCSK9 inhibitor therapies. Eur. Heart J. 39, 2546–2550 (2017).
Anderson, T. J. et al. 2016 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 32, 1263–1282 (2016).
Landmesser, U. et al. 2017 update of ESC/EAS task force on practical clinical guidance for proprotein convertase subtilisin/kexin type 9 inhibition in patients with atherosclerotic cardiovascular disease or in familial hypercholesterolaemia. Eur. Heart J. 39, 1131–1143 (2017).
Jaspers, N. E. M., Ridker, P. M., Dorresteijn, J. A. N. & Visseren, F. L. J. The prediction of therapy-benefit for individual cardiovascular disease prevention: rationale, implications, and implementation. Curr. Opin. Lipidol. 29, 436–444 (2018).
Nordestgaard, B. G. et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 37, 1944–1958 (2016).
Langlois, M. R. et al. Quantifying atherogenic lipoproteins: current and future challenges in the era of personalized medicine and VLDL cholesterol. A consensus statement from EAS and EFLM. Clin. Chem. 64, 1006–1033 (2018).
Martin, S. S. et al. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: insights from the FOURIER trial. JAMA Cardiol. 3, 749–753 (2018).
Sathiyakumar, V. et al. Impact of novel low-density lipoprotein-cholesterol assessment on the utility of secondary non-high-density lipoprotein-c and apolipoprotein B targets in selected worldwide dyslipidemia guidelines. Circulation 138, 244–254 (2018).
Pencina, K. M. et al. Trajectories of non–HDL cholesterol across midlife: implications for cardiovascular prevention. J. Am. Coll. Cardiol. 74, 70–79 (2019).
Eckel, R. H. et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129, S76–S99 (2014).
Arroyo-Olivares, R. et al. Adults with familial hypercholesterolaemia have healthier dietary and lifestyle habits compared with their non-affected relatives: the SAFEHEART study. Public Health Nutr. 22, 1433–1443 (2019).
Sacks, F. M. et al. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 136, e1–e23 (2017).
Gidding, S. S. Special commentary: is diet management helpful in familial hypercholesterolemia? Curr. Opin. Clin. Nutr. Metab. Care 22, 135–140 (2019).
Khera, A. V. et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N. Engl. J. Med. 375, 2349–2358 (2016).
Said, M. A., Verweij, N. & van der Harst, P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol. 3, 693–702 (2018).
American Diabetes Association. Standards of medical care in diabetes—2019 abridged for primary care providers. Clin. Diabetes 37, 11–34 (2019).
International Aspirin Foundation. Summary of UK guidelines for aspirin. Aspirin Foundation https://www.aspirin-foundation.com/guidelines/uk-guidelines-aspirin/ (2019).
Eikelboom, J. W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017).
Raal, F. J., Hovingh, G. K. & Catapano, A. L. Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis 277, 483–492 (2018).
Kusters, D. M. et al. Ten-year follow-up after initiation of statin therapy in children with familial hypercholesterolemia. JAMA 312, 1055–1057 (2014).
Humphries, S. et al. Coronary heart disease mortality in treated familial hypercholesterolaemia: update of the UK Simon Broome FH Register. Atherosclerosis 274, 41–46 (2018).
Nayak, A. et al. Legacy effects of statins on cardiovascular and all-cause mortality: a meta-analysis. BMJ Open 8, e020584 (2018).
Bos, S. et al. Carotid artery plaques and intima medial thickness in familial hypercholesteraemic patients on long-term statin therapy: a case control study. Atherosclerosis 256, 62–66 (2017).
Perez de Isla, L. et al. Long-term effect of 2 intensive statin regimens on treatment and incidence of cardiovascular events in familial hypercholesterolemia: the SAFEHEART study. J. Clin. Lipidol. 13, 989–996 (2019).
Ference, B. A. et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease. A Mendelian randomization analysis. J. Am. Coll. Cardiol. 60, 2631–2639 (2012).
Naito, R., Miyauchi, K. & Daida, H. Racial differences in the cholesterol-lowering effect of statin. J. Atheroscler. Thromb. 24, 19–25 (2017).
Tomlinson, B., Chan, P. & Liu, Z.-M. Statin responses in Chinese patients. J. Atheroscler. Thromb. 25, 199–202 (2018).
Taguchi, I. et al. High-dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD) a randomized superiority trial. Circulation 137, 1997–2009 (2018).
Hartgers, M. L. et al. Achieved LDL cholesterol levels in patients with heterozygous familial hypercholesterolemia: a model that explores the efficacy of conventional and novel lipid-lowering therapy. J. Clin. Lipidol. 12, 972–980.e1 (2018).
Cannon, C. P. et al. Ezetimibe added to statin therapy after acute coronary syndromes. N. Engl. J. Med. 372, 2387–2397 (2015).
Tsujita, K. et al. Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J. Am. Coll. Cardiol. 66, 495–507 (2015).
Watts, G. F., Pang, J., Chan, D. C., Brunt, J. N. & Lewis, B. Angiographic progression of coronary atherosclerosis in patients with familial hypercholesterolaemia treated with non-statin therapy: impact of a fat-modified diet and a resin. Atherosclerosis 252, 82–87 (2016).
Silverman, M. G. et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 316, 1289–1297 (2016).
Giugliano, R. P. et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation 137, 1571–1582 (2018).
Bach, R. G. et al. Effect of simvastatin-ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol. 4, 846–854 (2019).
Myocardial Infarction Genetics Consortium Investigators. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 371, 2072–2082 (2014).
Koopal, C. et al. Predicting the effect of fenofibrate on cardiovascular risk for individual patients with type 2 diabetes. Diabetes Care 41, 1244–1250 (2018).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Sahebkar, A., Reiner, Ž., Simental-Mendía, L. E., Ferretti, G. & Cicero, A. F. Effect of extended-release niacin on plasma lipoprotein (a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism 65, 1664–1678 (2016).
Burgess, S. et al. Association of LPA variants with risk of coronary disease and the implications for lipoprotein (a)-lowering therapies: a mendelian randomization analysis. JAMA Cardiol. 3, 619–627 (2018).
Yamashita, S. et al. Rationale and design of the PROSPECTIVE trial: probucol trial for secondary prevention of atherosclerotic events in patients with prior coronary heart disease. J. Atheroscler. Thromb. 23, 746–756 (2016).
Hartgers, M. L. et al. Alirocumab efficacy in patients with double heterozygous, compound heterozygous, or homozygous familial hypercholesterolemia. J. Clin. Lipidol. 12, 390–396.e8 (2018).
Ward, N. C., Page, M. M. & Watts, G. F. PCSK9 inhibition 2018: riding a new wave of coronary prevention. Clin. Sci. 133, 205–224 (2019).
Stoekenbroek, R. M., Lambert, G., Cariou, B. & Hovingh, G. K. Inhibiting PCSK9 — biology beyond LDL control. Nat. Rev. Endocrinol. 15, 52–62 (2019).
Defesche, J. C. et al. Efficacy of alirocumab in 1191 patients with a wide spectrum of mutations in genes causative for familial hypercholesterolemia. J. Clin. Lipidol. 11, 1338–1346.e7 (2017).
Raal, F. J. et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 385, 341–350 (2014).
Thedrez, A. et al. Homozygous familial hypercholesterolemia patients with identical mutations variably express the LDLR (low-density lipoprotein receptor) - implications for the efficacy of evolocumab. Arterioscler. Thromb. Vasc. Biol. 38, 592–598 (2018).
Moriarty, P. M. et al. Alirocumab in patients with heterozygous familial hypercholesterolemia undergoing lipoprotein apheresis: the ODYSSEY ESCAPE trial. Eur. Heart J. 37, 3588–3595 (2016).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Schwartz, G. G. et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107 (2018).
O’Donoghue, M. L. et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 139, 1483–1492 (2019).
Sabatine, M. S. et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease: an analysis from FOURIER. Circulation 138, 756–766 (2018).
Pérez de Isla, L. et al. Potential utility of the SAFEHEART risk equation for rationalising the use of PCSK9 monoclonal antibodies in adults with heterozygous familial hypercholesterolemia. Atherosclerosis 286, 40–45 (2019).
Kaasenbrood, L. et al. Estimated individual lifetime benefit from PCSK9 inhibition in statin-treated patients with coronary artery disease. Heart 104, 1699–1705 (2018).
Navarese, E. P. et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA 319, 1566–1579 (2018).
Hlatky, M. A. & Kazi, D. S. PCSK9 inhibitors: economics and policy. J. Am. Coll. Cardiol. 70, 2677–2687 (2017).
Stoekenbroek, R. M. et al. PCSK9 inhibitors in clinical practice: delivering on the promise? Atherosclerosis 270, 205–210 (2018).
Gürgöze, M. T. et al. Adverse events associated with PCSK 9 inhibitors: a real-world experience. Clin. Pharmacol. Ther. 105, 496–504 (2019).
Baum, S. J. & Brown, A. S. Familial hypercholesterolemia: although identification advances, appreciation and treatment lag. Rev. Cardiovasc. Med. 19, S25–S30 (2018).
Myers, K. D. et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ. Cardiovasc. Qual. Outcomes 12, e005404 (2019).
Bellosta, S. & Corsini, A. Statin drug interactions and related adverse reactions: an update. Expert. Opin. Drug. Saf. 17, 25–37 (2018).
Newman, C. B. et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 39, e38–e81 (2019).
Mach, F. et al. Adverse effects of statin therapy: perception vs. the evidence–focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 39, 2526–2539 (2018).
Kajinami, K. et al. Statin intolerance clinical guide 2018. J. Atheroscler. Thromb. https://doi.org/10.5551/jat.50948 (2019).
Lotta, L. A. et al. Association between low-density lipoprotein cholesterol–lowering genetic variants and risk of type 2 diabetes: a meta-analysis. JAMA 316, 1383–1391 (2016).
Fuentes, F., Alcalá-Díaz, J. F., Watts, G. F. & Mata, P. Diabetes, statins and FH. Int. J. Cardiol. 203, 575 (2016).
Besseling, J., Kastelein, J. J., Defesche, J. C., Hutten, B. A. & Hovingh, G. K. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA 313, 1029–1036 (2015).
Tsimikas, S., Gordts, P. L., Nora, C., Yeang, C. & Witztum, J. L. Statin therapy increases lipoprotein (a) levels. Eur. Heart J. https://doi.org/10.1093/eurheartj/ehz310 (2019).
Ward, N. C., Watts, G. F. & Eckel, R. H. Statin toxicity: mechanistic insights and clinical implications. Circ. Res. 124, 328–350 (2019).
Stroes, E. S. et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur. Heart J. 36, 1012–1022 (2015).
Rosenson, R. S. et al. Optimizing cholesterol treatment in patients with muscle complaints. J. Am. Coll. Cardiol. 70, 1290–1301 (2017).
Olsson, A. G. et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. J. Intern. Med. 281, 534–553 (2017).
Rosenson, R. S., Hegele, R. A. & Koenig, W. Cholesterol-lowering agents: PCSK9 inhibitors today and tomorrow. Circ. Res. 124, 364–385 (2019).
Nissen, S. E. et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 315, 1580–1590 (2016).
Moriarty, P. M. et al. Efficacy and safety of alirocumab vs ezetimibe in statin-intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J. Clin. Lipidol. 9, 758–769 (2015).
Sun, L. et al. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat. Med. 25, 569 (2019).
Serban, M.-C. et al. Statin intolerance and risk of coronary heart events and all-cause mortality following myocardial infarction. J. Am. Coll. Cardiol. 69, 1386–1395 (2017).
Nielsen, S. F. & Nordestgaard, B. G. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur. Heart J. 37, 908–916 (2015).
Aggarwal, N. R. et al. Sex differences in ischemic heart disease: advances, obstacles, and next steps. Circ. Cardiovasc. Qual. Outcomes 11, e004437 (2018).
Amrock, S. M. et al. Health disparities among adult patients with a phenotypic diagnosis of familial hypercholesterolemia in the CASCADE-FH™ patient registry. Atherosclerosis 267, 19–26 (2017).
Jacobson, T. A. et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: part 2. J. Clin. Lipidol. 9, S1–S122.e1 (2015).
Regitz-Zagrosek, V. et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 39, 3165–3241 (2018).
Lameijer, H. et al. Pregnancy in women with pre-existent ischaemic heart disease: a systematic review with individualised patient data. Heart 105, 873–880 (2019).
Stefanutti, C. et al. Toward an international consensus—integrating lipoprotein apheresis and new lipid-lowering drugs. J. Clin. Lipidol. 11, 858–871.e3 (2017).
France, M. et al. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis 255, 128–139 (2016).
Li, W., Ruan, W., Lu, Z. & Wang, D. Parity and risk of maternal cardiovascular disease: a dose-response meta-analysis of cohort studies. Eur. J. Prev. Cardiol. 26, 592–602 (2019).
Smith, C. J. et al. Maternal dyslipidemia and risk for preterm birth. PLOS ONE 13, e0209579 (2018).
Toleikyte, I., Retterstøl, K., Leren, T. P. & Iversen, P. O. Pregnancy outcomes in familial hypercholesterolemia - a registry-based study. Circulation 124, 1606–1614 (2011).
Pieper, P. G. Use of medication for cardiovascular disease during pregnancy. Nat. Rev. Cardiol. 12, 718–729 (2015).
Winterfeld, U. et al. Pregnancy outcome following maternal exposure to statins: a multicentre prospective study. BJOG 120, 463–471 (2013).
Botha, T. C., Pilcher, G. J., Wolmarans, K., Blom, D. J. & Raal, F. J. Statins and other lipid-lowering therapy and pregnancy outcomes in homozygous familial hypercholesterolaemia: a retrospective review of 39 pregnancies. Atherosclerosis 277, 502–507 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02957604 (2019).
Braamskamp, M. J. A. M. et al. Efficacy and safety of rosuvastatin therapy in children and adolescents with familial hypercholesterolemia: results from the CHARON study. J. Clin. Lipidol. 9, 741–750 (2015).
Lázaro, P. et al. Cost-effectiveness of a cascade screening program for the early detection of familial hypercholesterolemia. J. Clin. Lipidol. 11, 260–271 (2017).
Pelczarska, A. et al. The cost-effectiveness of screening strategies for familial hypercholesterolaemia in Poland. Atherosclerosis 270, 132–138 (2018).
Benn, M., Tybjærg-Hansen, A. & Nordestgaard, B. G. Low LDL cholesterol by PCSK9 variation reduces cardiovascular mortality. J. Am. Coll. Cardiol. 73, 3102–3114 (2019).
de Ferranti, S. D. et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association. Circulation 139, e603–e634 (2019).
Ramaswami, U., Cooper, J. & Humphries, S. E. The UK paediatric familial hypercholesterolaemia register: preliminary data. Arch. Dis. Child. 102, 255–260 (2016).
Humphries, S. E., Cooper, J., Dale, P. & Ramaswami, U. The UK paediatric familial hypercholesterolaemia register: statin-related safety and 1-year growth data. J. Clin. Lipidol. 12, 25–32 (2018).
Vallejo-Vaz, A. J. et al. Pooling and expanding registries of familial hypercholesterolaemia to assess gaps in care and improve disease management and outcomes: rationale and design of the global EAS familial hypercholesterolaemia studies collaboration. Atheroscler. Suppl. 22, 1–32 (2016).
Bellgard, M. I. et al. Design of the familial hypercholesterolaemia Australasia Network Registry: creating opportunities for greater international collaboration. J. Atheroscler. Thromb. 24, 1075–1084 (2017).
Ramaswami, U. et al. Current management of children and young people with heterozygous familial hypercholesterolaemia - HEART UK statement of care. Atherosclerosis 290, 1–8 (2019).
Harada-Shiba, M. et al. Efficacy and safety of pitavastatin in children and adolescents with familial hypercholesterolemia in Japan and Europe. J. Atheroscler. Thromb. 25, 422–429 (2018).
Vuorio, A. et al. Statins for children with familial hypercholesterolemia. Cochrane Database Syst. Rev. 11, CD006401 (2019).
Mamann, N. et al. Intermediate-term efficacy and tolerance of statins in children. J. Pediatr. 210, 161–165 (2019).
Kusters, D. M. et al. Efficacy and safety of ezetimibe monotherapy in children with heterozygous familial or nonfamilial hypercholesterolemia. J. Pediatr. 166, 1377–1384.e3 (2015).
Sliwinski, S. K. et al. Transitioning from pediatric to adult health care with familial hypercholesterolemia: listening to young adult and parent voices. J. Clin. Lipidol. 11, 147–159 (2017).
Stein, E. A. et al. Efficacy of rosuvastatin in children with homozygous familial hypercholesterolemia and association with underlying genetic mutations. J. Am. Coll. Cardiol. 70, 1162–1170 (2017).
Gaudet, D. et al. Efficacy, safety, and tolerability of evolocumab in pediatric patients with heterozygous familial hypercholesterolemia: rationale and design of the HAUSER-RCT study. J. Clin. Lipidol. 12, 1199–1207 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02624869 (2019).
Ben-Omran, T. et al. Real-world outcomes with lomitapide use in paediatric patients with homozygous familial hypercholesterolaemia. Adv. Ther. 36, 1786–1811 (2019).
Luirink, I. K. et al. Coronary computed tomography angiography and echocardiography in children with homozygous familial hypercholesterolemia. Atherosclerosis 285, 87–92 (2019).
Luirink, I. K. et al. Efficacy and safety of lipoprotein apheresis in children with homozygous familial hypercholesterolemia: a systematic review. J. Clin. Lipidol. 13, 31–39 (2019).
Thompson, G. & Parhofer, K. G. Current role of lipoprotein apheresis. Curr. Atheroscler. Rep. 21, 26 (2019).
Roeseler, E. et al. Lipoprotein apheresis for lipoprotein(a)-associated cardiovascular disease: prospective 5 years of follow-up and apo(a) characterization. Arterioscler. Thromb. Vasc. Biol. 36, 2019–2027 (2016).
Drouin-Chartier, J.-P., Tremblay, A. J., Bergeron, J., Lamarche, B. & Couture, P. The low-density lipoprotein receptor genotype is a significant determinant of the rebound in low-density lipoprotein cholesterol concentration after lipoprotein apheresis among patients with homozygous familial hypercholesterolemia. Circulation 136, 880–882 (2017).
Thompson, G. R. et al. Survival in homozygous familial hypercholesterolaemia is determined by the on-treatment level of serum cholesterol. Eur. Heart J. 39, 1162–1168 (2017).
Bruckert, E. et al. Long-term outcome in 53 patients with homozygous familial hypercholesterolaemia in a single centre in France. Atherosclerosis 257, 130–137 (2017).
Stefanutti, C. et al. A cross-national investigation of cardiovascular survival in homozygous familial hypercholesterolemia: the Sino-Roman study. J. Clin. Lipidol. 13, 608–617 (2019).
Ishigaki, Y. et al. Liver transplantation for homozygous familial hypercholesterolemia. J. Atheroscler. Thromb. 26, 121–127 (2019).
Martinez, M. et al. Effects of liver transplantation on lipids and cardiovascular disease in children with homozygous familial hypercholesterolemia. Am. J. Cardiol. 118, 504–510 (2016).
Hegele, R. A. & Tsimikas, S. Lipid-lowering agents: targets beyond PCSK9. Circ. Res. 124, 386–404 (2019).
Ray, K. K. et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 376, 1430–1440 (2017).
Gaudet, D. et al. Usefulness of gemcabene in homozygous familial hypercholesterolemia (from COBALT-1). Am. J. Cardiol. 124, 1876–1880 (2019).
Fruchart, J.-C. et al. The selective peroxisome proliferator-activated receptor alpha modulator (SPPARMα) paradigm: conceptual framework and therapeutic potential. Cardiovasc. Diabetol. 18, 71 (2019).
Gaudet, D. et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N. Engl. J. Med. 377, 296–297 (2017).
Ajufo, E. & Cuchel, M. Recent developments in gene therapy for homozygous familial hypercholesterolemia. Curr. Atheroscler. Rep. 18, 22 (2016).
Tsimikas, S. RNA-targeted therapeutics for lipid disorders. Curr. Opin. Lipidol. 29, 459–466 (2018).
Graham, I. et al. New strategies for the development of lipid-lowering therapies to reduce cardiovascular risk. Eur. Heart J. Cardiovasc. Pharmacother. 4, 119–127 (2017).
Currie, G. & Delles, C. Precision medicine and personalized medicine in cardiovascular disease. Adv. Exp. Med. Biol. 1065, 589–605 (2018).
Karimi-Shahanjarini, A. et al. Barriers and facilitators to the implementation of doctor-nurse substitution strategies in primary care: a qualitative evidence synthesis. Cochrane Database Syst. Rev. 5, CD010412 (2019).
Warden, B. A., Shapiro, M. D. & Fazio, S. The role of the clinical pharmacist in a preventive cardiology practice. Ann. Pharmacother. 53, 1214–1219 (2019).
Cooke, J. A framework to evaluate research capacity building in health care. BMC Fam. Pract. 6, 44 (2005).
Peters, D. H., Tran, N. T. & Adam, T. Implementation research health: a practical guide. https://apps.who.int/iris/bitstream/handle/10665/91758/9789241506212_eng.pdf (WHO, 2013).
Carman, K. L. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff. 32, 223–231 (2013).
Payne, J. et al. Familial hypercholesterolaemia patient support groups and advocacy: a multinational perspective. Atherosclerosis 277, 377–382 (2018).
Bangash, H., Khan, F., He, B., Arce, M. & Kullo, I. J. Use of Twitter to promote awareness of familial hypercholesterolemia. Circ. Genom. Precis. Med. 12, e002550 (2019).
Baum, S. J. et al. PCSK9 inhibitor access barriers—issues and recommendations: improving the access process for patients, clinicians and payers. Clin. Cardiol. 40, 243–254 (2017).
Jones, L. K. et al. Healthcare utilization and patients’ perspectives after receiving a positive genetic test for familial hypercholesterolemia. Circ. Genom. Precis. Med. 11, e002146 (2018).
World Health Organization. Familial hypercholesterolaemia (FH): report of a WHO consultation. https://apps.who.int/iris/bitstream/handle/10665/64162/WHO_HGN_FH_CONS_98.7.pdf (WHO, 1997).
Bufalino, V. J. et al. The American Heart Association’s recommendations for expanding the applications of existing and future clinical registries. Circulation 123, 2167–2179 (2011).
Mundal, L. J. et al. Impact of age on excess risk of coronary heart disease in patients with familial hypercholesterolaemia. Heart 104, 1600–1607 (2018).
Ruel, I. et al. Simplified Canadian definition for familial hypercholesterolemia. Can. J. Cardiol. 34, 1210–1214 (2018).
Pang, J. et al. Comparative aspects of the care of familial hypercholesterolemia in the “Ten Countries Study”. J. Clin. Lipidol. 13, 287–300 (2019).
Barton Duell, P. et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: the CASCADE FH registry. Atherosclerosis 289, 85–93 (2019).
Saltijeral, A. et al. Attainment of LDL cholesterol treatment goals in children and adolescents with familial hypercholesterolemia: the SAFEHEART follow-up registry. Rev. Esp. Cardiol. 70, 444–450 (2017).
Ellis, K. L., Pang, J. & Watts, G. F. Registries, codifications and cardiovascular outcomes in familial hypercholesterolaemia. Eur. J. Prev. Cardiol. 24, 133–136 (2016).
Gee, M. & Cooke, J. How do NHS organisations plan research capacity development? Strategies, strengths, and opportunities for improvement. BMC Health Serv. Res. 18, 198 (2018).
Martin, A. C., Gidding, S. S., Wiegman, A. & Watts, G. F. Known and unknowns in the care of paediatric familial hypercholesterolaemia. J. Lipid Res. 58, 1765–1776 (2017).
Pang, J., Lansberg, P. J. & Watts, G. F. International developments in the care of familial hypercholesterolemia: where now and where to next? J. Atheroscler. Thromb. 23, 505–519 (2016).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br. Med. J. 336, 924–926 (2008).
Vallejo-Vaz, A. J. et al. Familial hypercholesterolaemia: a global call to arms. Atherosclerosis 243, 257–259 (2015).
deGoma, E. M. et al. Treatment gaps in adults with heterozygous familial hypercholesterolemia in the United States: data from the CASCADE-FH Registry. Circ. Cardiovasc. Genet. 9, 240–249 (2016).
Bauer, M. S., Damschroder, L., Hagedorn, H., Smith, J. & Kilbourne, A. M. An introduction to implementation science for the non-specialist. BMC Psychol. 3, 32 (2015).
Chambers, D. A., Feero, W. G. & Khoury, M. J. Convergence of implementation science, precision medicine, and the learning health care system: a new model for biomedical research. JAMA 315, 1941–1942 (2016).
Glasgow, R. E., Vogt, T. M. & Boles, S. M. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am. J. Public Health 89, 1322–1327 (1999).
Feldstein, A. C. & Glasgow, R. E. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt. Comm. J. Qual. Patient Saf. 34, 228–243 (2008).
Jones, L. K. et al. Developing implementation strategies to improve uptake of guideline-recommended treatments for individuals with familial hypercholesterolemia: a protocol. Res. Soc. Adm. Pharm. https://doi.org/10.1016/j.sapharm.2019.06.006 (2019).
World Health Organization. Familial hypercholesterolaemia (FH): report of a second WHO consultation. https://apps.who.int/iris/bitstream/handle/10665/66346/WHO_HGN_FH_CONS_99.2.pdf (WHO, 1999).
Author information
Authors and Affiliations
Contributions
G.F.W. and J.P. researched data for the article, and all authors contributed to discussions of its content. G.F.W. wrote the manuscript, and all authors reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors received no financial support for the research, authorship or publication of this article. G.F.W. has received honoraria as a consultant on advisory boards and research grants from Amgen, Regeneron Pharmaceuticals and Sanofi. S.S.G. has received research grants from the US National Institutes of Health and is employed by the FH Foundation. P.M. has received research grants from Amgen and Sanofi. J.P. was supported by a WAHTN Early Career Fellowship and the Australian Government’s Medical Research Future Fund. D.R.S. has received grants from Amarin, Amgen, AstraZeneca, Espirion, Novartis and Regeneron Pharmaceuticals, as well as personal fees from Amgen and Sanofi. S.Y. has received grants and personal fees from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Daiichi Sankyo, Hayashibara, Izumisano City, Japan Tobacco, Kaizuka City, Kaken Pharmaceutical, Kissei Pharmaceutical, Kowa, Kyowa Medex, Merck Sharp & Dohme, Mochida Pharmaceutical, the Japanese National Institute of Biomedical Innovation, Nippon Boehringer Ingelheim, Otsuka Pharmaceutical, Sanwa Kagaku Kenkyusho, Shionogi, Takeda Pharmaceutical and Teijin Pharma, as well as personal fees from Amgen Astellas BioPharma, Astellas Pharma, AstraZeneca, Bayer Yakuhin, Bristol-Myers Squibb, Daiichi Sankyo, Kaken Pharmaceutical, Medical Review, Merck Sharp & Dohme, Ono Pharmaceutical, Otsuka Pharmaceutical, Pfizer Japan, Sanofi, Sanwa Kagaku Kenkyusho, Shionogi, Skylight Biotech, Takeda Pharmaceutical and Toa Eiyo. F.J.R. has received research grants from Amgen, Sanofi and Regeneron Pharmaceuticals, has participated in speakers’ bureaus for and received honoraria from Amgen, Regeneron Pharmaceuticals, Sanofi and The Medicines Company, and is a consultant on advisory boards for Amgen, Regeneron Pharmaceuticals, Sanofi and The Medicines Company. R.D.S. is a recipient of a scholarship from the Conselho Nacional de Pesquisa e Desenvolvimento Tecnologico (CNPq) process no. 303734/2018-3 and has received honoraria for consulting, research and speaker activities from Ache, Akcea, Amgen, AstraZeneca, Esperion, Kowa, Merck, Novo-Nordisk, Pfizer and Sanofi/Regeneron Pharmaceuticals. K.K.R. has received research grants from Amgen, Merck Sharp & Dohme, Pfizer, Regeneron and Sanofi, as well as honoraria for lectures, being a consultant on advisory boards and/or as a steering committee member from Amgen, AstraZeneca, Boehringer Ingelheim, Esperion, IONIS, Kowa, Lilly, Pfizer, Regeneron Pharmaceuticals, Sanofi, Takeda and The Medicines Company.
Additional information
Peer review information
Nature Reviews Cardiology thanks R. Hegele, L. Ose and E. Stein for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria
A search was undertaken of the literature published in the English language between January 2014 and September 2019. The search used the PubMed database with search string (all fields) ‘familial hypercholesterolemia’ or ‘familial hypercholesterolaemia’. Additional published studies were provided ad hoc by individual authors. G.F.W. and J.P. assessed the titles and abstracts of all the articles identified and selected those that were novel and most useful for informing the components of the model of care for familial hypercholesterolaemia. The other authors approved this selection and supplied additional articles that added value to those identified in the literature review.
Related links
ClinVar database: https://www.ncbi.nlm.nih.gov/clinvar/
European Atherosclerosis Society Familial Hypercholesterolaemia Studies Collaboration: https://www.eas-society.org/page/fhsc
European FH Patient Network: https://fheurope.org/
FH Foundation: https://thefhfoundation.org/
Rights and permissions
About this article
Cite this article
Watts, G.F., Gidding, S.S., Mata, P. et al. Familial hypercholesterolaemia: evolving knowledge for designing adaptive models of care. Nat Rev Cardiol 17, 360–377 (2020). https://doi.org/10.1038/s41569-019-0325-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0325-8
This article is cited by
-
Screening and verifying the mutations in the LDLR and APOB genes in a Chinese family with familial hypercholesterolemia
Lipids in Health and Disease (2023)
-
Metabolic systems approaches update molecular insights of clinical phenotypes and cardiovascular risk in patients with homozygous familial hypercholesterolemia
BMC Medicine (2023)
-
Stratification in Heterozygous Familial Hypercholesterolemia: Imaging, Biomarkers, and Genetic Testing
Current Atherosclerosis Reports (2023)
-
International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia
Nature Reviews Cardiology (2023)
-
Apolipoproteins in vascular biology and atherosclerotic disease
Nature Reviews Cardiology (2022)