Abstract
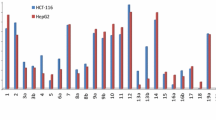
New series of 2,4,6-triarylpyridines derivatives 5a–f and 6a–d were designed, synthesized and evaluated for their cytotoxic activity against breast cancer cell lines. Three-component reaction between acetophenones, ammonium acetate and 4-methyl or 2-methylpyridine in the presence of iodine in DMSO lead to title 2,4,6-triarylpyridines. This method allows the simple preparation of the desired products using readily available reagents under mild reaction conditions in good yields. Synthesized compounds were investigated for their cytotoxic activities against MDA-MB-231, MCF-7 and T-47D cell lines. Most of the synthesized compounds showed remarkable cytotoxicity, comparable to standard drug etoposide.




Similar content being viewed by others
References
G.D. Henry, Tetrahedron 60, 6043 (2004)
M. Borthakur, M. Dutta, S. Gogoi, R.C. Boruah, Synlett 2008, 3125 (2008)
I.J. Enyedy, S. Sakamuri, W.A. Zaman, K.M. Johnson, S. Wang, Bioorg. Med. Chem. Lett. 13, 513 (2003)
A. Shrestha, S. Park, S. Shin, T.M. Kadayat, G. Bist, P. Katila, Y. Kwon, E.S. Lee, Bioorg. Chem. 79, 1 (2018)
Y. Asanuma, H. Eguchi, H. Nishiyama, I. Tomita, S. Inagi, Org. Lett. 19, 1824 (2017)
J. Marquet, M. Moreno-Manas, P. Pacheco, M. Prat, A.R. Katritzky, B. Brycki, Tetrahedron 46, 5333 (1990)
R.A. Abramovitch, J.M. Beckert, P. Chinnasamy, H. Xiaohua, W. Pennington, A.R.V. Sanjivamurthy, Heterocycles 28, 623 (1989)
A.R. Katritzky, J.M. Aurrecoechea, K.K. Quian, E. Anna, G.J. Palenik, Heterocycles 25, 387 (1987)
R.M. Martin, R.G. Bergman, J.A. Ellman, J. Org. Chem. 77, 2501 (2012)
J.M. Neely, T. Rovis, J. Am. Chem. Soc. 135, 66 (2013)
J. Wu, W. Xu, Z.X. Yu, J. Wang, J. Am. Chem. Soc. 137, 9489 (2015)
X. Zhang, Z. Wang, K. Xu, Y. Feng, W. Zhao, X. Xu, Y. Yan, W. Yi, Green Chem. 18, 2313 (2016)
R.S. Rohokale, B. Koenig, D.D. Dhavale, J. Org. Chem. 81, 7121 (2016)
R. Karkia, R.K.Y. Junb, T.M. Kadayat, S. Shin, T.B.T. Magar, G. Bist, A. Shrestha, Y. Na, Y. Kwon, E.S. Lee, Eur. J. Med. Chem. 113, 228 (2016)
R. Karkia, C. Song, T.M. Kadayat, T.B.T. Magar, G. Bist, A. Shrestha, Y. Na, Y. Kwon, E.S. Lee, Bioorg. Med. Chem. 23, 3638 (2015)
R. Karkia, C. Park, K.Y. Jun, J.G. Jee, J.H. Lee, P. Thapa, T.M. Kadayat, Y. Kwon, E.S. Lee, Eur. J. Med. Chem. 84, 555 (2014)
F. Kröhnke, W. Zecher, J. Curtze, D. Drechsler, K. Pfleghar, K.E. Schnalke, W. Weis, Angew. Chem. Int. Ed. 1, 626 (1962)
F. Kröhnke, Synthesis 1, 1 (1976)
K.T. Potts, M.J. Cipullo, P. Ralli, G. Theodoridis, J. Am. Chem. Soc. 103, 3584 (1981)
T. Kobayashi, H. Kakiuchi, H. Kato, Bull. Chem. Soc. Jpn 64, 392 (1991)
F. Palacios, A.M.O. de Retana, J. Oyarzabal, Tetrahedron Lett. 37, 4577 (1996)
G.W.V. Cave, C.L. Raston, Chem. Commun. 22, 2199 (2000)
S. Tu, T. Li, F. Shi, F. Fang, S. Zhu, X. Wei, Z. Zong, Chem. Lett. 34, 732 (2005)
J.C. Xiang, Y. Cheng, Z.X. Wang, J.T. Ma, M. Wang, B.C. Tang, Y.D. Wu, A.X. Wu, Org. Lett. 19, 2997 (2017)
Z. Tashrifi, M. Mohammadi-khanaposhtani, M. Shafiee Ardestani, M. Safavi, K. Rad-Moghadam, M. Mehrdad, B. Larijani, M. Mahdavi, Lett. Drug Des. Discov. 6, 213 (2019)
F. Khalili, S. Akrami, M. Safavi, M. Mohammadi-Khanaposhtani, M. Saeedi, S.K. Ardestani, B. Larijani, A. Zonouzi, M.B. Tehrani, M. Mahdavi, Anti-Cancer Agents Med. Chem. 19, 265 (2019)
M. Nikpassand, L. Zare Fekri, M. Nabatzadeh, Comb. Chem. High Throughput Screen. 20, 533 (2017)
M. Nikpassand, L. Zare Fekri, S. Sanagou, Dye. Pigment. 136, 140 (2017)
H. Taherkhorsand, M. Nikpassand, Comb. Chem. High Throughput Screen. 21, 65 (2018)
M. Nikpassand, D. Pirdelzendeh, Dye. Pigment. 130, 314 (2016)
M. Nikpassand, L. Zare Fekri, P. Farokhian, Ultrason. Sonochem. 28, 341 (2016)
M. Nikpassand, L. Zare Fekri, L. Karimian, M. Rassa, Curr. Org. Synth. 12, 358 (2015)
L. Zare Fekri, M. Nikpassand, S. Pourmirzajani, B. Aghazadeh, RSC Adv. 8, 22313 (2018)
M. Mohammadi-Khanaposhtani, S. Rezaei, R. Khalifeh, S. Imanparast, M.A. Faramarzi, S. Bahadorikhalili, M. Safavi, F. Bandarian, E.N. Esfahani, M. Mahdavi, B. Larijani, Bioorg. Chem. 80, 288 (2018)
M. Mohammadi-Khanaposhtani, K. Fahimi, E. Karimpour-Razkenari, M. Safavi, M. Mahdavi, M. Saeedi, T. Akbarzadeh, Lett. Drug Des. Discov. 16, 818 (2019)
M. Mohammadi-Khanaposhtani, M. Safavi, R. Sabourian, M. Mahdavi, M. Pordeli, M. Saeedi, S.K. Ardestani, A. Foroumadi, A. Shafiee, T. Akbarzadeh, Mol. Divers. 19, 787 (2015)
R. Karkia, C. Park, K.Y. Jun, J.G. Jee, J.H. Lee, P. Thapa, T.M. Kadayat, Y. Kwon, E.S. Lee, Euro. J. Med. Chem. 84, 555 (2014)
Acknowledgements
Financial support from the Research Council of Islamic Azad University of Rasht branch is sincerely acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baloutaki, B.A., Sayahi, M.H., Nikpassand, M. et al. An efficient method for the synthesis of new derivatives of 2,4,6-triarylpyridines as cytotoxic agents. Res Chem Intermed 46, 1153–1163 (2020). https://doi.org/10.1007/s11164-019-04025-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04025-6