Abstract
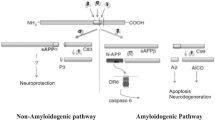
Amyloid precursor protein (APP) is a transmembrane protein expressed largely within the central nervous system. Upon cleavage, it does not produce the toxic amyloid peptide (Aβ) only, which is involved in neurodegenerative progressions but via a non-amyloidogenic pathway it is metabolized to produce a soluble fragment (sAPPα) through α-secretase. While a lot of studies are focusing on the role played by APP in the pathogenesis of Alzheimer’s disease, sAPPα is reported to have numerous neuroprotective effects and it is being suggested as a candidate with possible therapeutic potential against Alzheimer’s disease. However, the mechanisms through which sAPPα precisely works remain elusive. We have presented a comprehensive review of how sAPPα is regulating the neuroprotective effects in different biological models. Moreover, we have focused on the role of sAPPα during different developmental stages of the brain, neurogenic microenvironment in the brain and how this metabolite of APP is regulating the neurogenesis which is regarded as a compelling approach to ameliorate the impaired learning and memory deficits in dementia and diseases like Alzheimer’s disease. sAPPα exerts beneficial physiological, biochemical and behavioral effects mitigating the detrimental effects of neurotoxic compounds. It has shown to increase the proliferation rate of numerous cell types and promised the synaptogenesis, neurite outgrowth, cell survival and cell adhesion. Taken together, we believe that further studies are warranted to investigate the exact mechanism of action so that sAPPα could be developed as a novel therapeutic target against neuronal deficits.


Similar content being viewed by others
Abbreviations
- APP:
-
Amyloid precursor protein
- sAPPα:
-
Secreted amyloid precursor protein alpha
- ADAM 10:
-
A disintegrin and metalloproteinase domain-containing protein 10
- BACE1:
-
Beta-secretase enzyme 1
- CCI:
-
Controlled cortical impact
- IDE:
-
Insulin degrading enzyme
- NSC:
-
Neural stem cell
- NMDA:
-
N-Methyl-d-aspartate
- SGZ:
-
Subgranular zone
- SVZ:
-
Sub-ventricular zone
- TBI:
-
Traumatic brain injury
References
Dorard E et al (2018) Soluble amyloid precursor protein alpha interacts with alpha3-Na, K-ATPAse to induce axonal outgrowth but not neuroprotection: evidence for distinct mechanisms underlying these properties. Mol Neurobiol 55:5594–5610
Taylor CJ et al (2008) Endogenous secreted amyloid precursor protein-α regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol Dis 31(2):250–260
Moreno L et al (2015) sAβPPα improves hippocampal NMDA-dependent functional alterations linked to healthy aging. J Alzheimers Dis 48(4):927–935
Ring S et al (2007) The secreted β-amyloid precursor protein ectodomain APPsα is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci 27(29):7817–7826
Fol R et al (2016) Viral gene transfer of APPsα rescues synaptic failure in an Alzheimer’s disease mouse model. Acta Neuropathol 131(2):247–266
Eriksson PS et al (1998) Neurogenesis in the adult human hippocampus. Nat Med 4(11):1313
Doetsch F et al (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97(6):703–716
Kempermann G, Song H, Gage FH (2015) Neurogenesis in the adult hippocampus. Cold Spring Harb Perspect Biol 7(9):a018812
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124(3):319–335
Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132(4):645–660
Alvarez-Buylla A, Garcıa-Verdugo JM (2002) Neurogenesis in adult subventricular zone. J Neurosci 22(3):629–634
Kempermann G, Wiskott L, Gage FH (2004) Functional significance of adult neurogenesis. Curr Opin Neurobiol 14(2):186–191
Babu H et al (2009) Synaptic network activity induces neuronal differentiation of adult hippocampal precursor cells through BDNF signaling. Front Neurosci 3:1
Sorrells SF et al (2018) Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555(7696):377
Boldrini M et al (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22(4):589–599
Tartt AN et al (2018) Considerations for assessing the extent of hippocampal neurogenesis in the adult and aging human brain. Cell Stem Cell 23(6):782–783
Moreno-Jiménez EP et al (2019) Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat Med 25(4):554
Plant LD et al (2003) The production of amyloid β peptide is a critical requirement for the viability of central neurons. J Neurosci 23(13):5531–5535
López-Toledano MA, Shelanski ML (2004) Neurogenic effect of β-amyloid peptide in the development of neural stem cells. J Neurosci 24(23):5439–5444
Heo C et al (2007) Effects of the monomeric, oligomeric, and fibrillar Aβ42 peptides on the proliferation and differentiation of adult neural stem cells from subventricular zone. J Neurochem 102(2):493–500
Chen Y, Dong C (2009) Aβ40 promotes neuronal cell fate in neural progenitor cells. Cell Death Differ 16(3):386
Zhou Z-D et al (2011) The roles of amyloid precursor protein (APP) in neurogenesis: implications to pathogenesis and therapy of Alzheimer disease. Cell Adhes Migr 5(4):280–292
Goldgaber D et al (1987) Isolation, characterization, and chromosomal localization of human brain cDNA clones coding for the precursor of the amyloid of brain in Alzheimer’s disease, Down’s syndrome and aging. J Neural Transm Suppl 24:23–28
Kang J et al (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325(6106):733
Wasco W et al (1992) Identification of a mouse brain cDNA that encodes a protein related to the Alzheimer disease-associated amyloid beta protein precursor. Proc Natl Acad Sci 89(22):10758–10762
Daigle I, Li CA (1993) apl-1, a Caenorhabditis elegans gene encoding a protein related to the human beta-amyloid protein precursor. Proc Natl Acad Sci 90(24):12045–12049
Rosen DR et al (1989) A Drosophila gene encoding a protein resembling the human beta-amyloid protein precursor. Proc Natl Acad Sci 86(7):2478–2482
Gralle M, Ferreira ST (2007) Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol 82(1):11–32
Yoshikai S-I et al (1990) Genomic organization of the human amyloid beta-protein precursor gene. Gene 87(2):257–263
Ponte P et al (1988) A new A4 amyloid mRNA contains a domain homologous to serine proteinase inhibitors. Nature 331(6156):525
Tanaka S et al (1988) Three types of amyloid protein precursor mRNA in human brain: their differential expression in Alzheimer’s disease. Biochem Biophys Res Commun 157(2):472–479
Haass C, Hung AY, Selkoe DJ (1991) Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci 11(12):3783–3793
Kitaguchi N et al (1988) Novel precursor of Alzheimer’s disease amyloid protein shows protease inhibitory activity. Nature 331(6156):530
König G et al (1992) Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J Biol Chem 267(15):10804–10809
Fan Y et al (2018) Does the genetic feature of the Chinese tree shrew (Tupaia belangeri chinensis) support its potential as a viable model for Alzheimer’s disease research? J Alzheimers Dis 61(3):1015–1028
Murrell J et al (1991) A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 254(5028):97–99
Wiseman FK et al (2015) A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome. Nat Rev Neurosci 16(9):564
Lee M-H et al (2018) Somatic APP gene recombination in Alzheimer’s disease and normal neurons. Nature 563(7733):639
Scheuermann S et al (2001) Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer’s disease. J Biol Chem 276(36):33923–33929
Soba P et al (2005) Homo-and heterodimerization of APP family members promotes intercellular adhesion. EMBO J 24(20):3624–3634
Peron R et al (2018) Alpha-secretase ADAM10 regulation: insights into Alzheimer’s disease treatment. Pharmaceuticals 11(1):12
Farzan M et al (2000) BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc Natl Acad Sci 97(17):9712–9717
Tanabe C et al (2007) ADAM19 is tightly associated with constitutive Alzheimer’s disease APP α-secretase in A172 cells. Biochem Biophys Res Commun 352(1):111–117
Asai M et al (2003) Putative function of ADAM9, ADAM10, and ADAM17 as APP α-secretase. Biochem Biophys Res Commun 301(1):231–235
Lopez Sanchez MIG, van Wijngaarden P, Trounce IA (2019) Amyloid precursor protein-mediated mitochondrial regulation and Alzheimer’s disease. Br J Pharmacol 176(18):3464–3474
Andrew RJ et al (2016) A Greek tragedy: the growing complexity of Alzheimer amyloid precursor protein proteolysis. J Biol Chem 291(37):19235–19244
Wang H et al (2015) Cathepsin L mediates the degradation of novel APP C-terminal fragments. Biochemistry 54(18):2806–2816
Willem M et al (2015) η-Secretase processing of APP inhibits neuronal activity in the hippocampus. Nature 526(7573):443
Laßek M et al (2013) Amyloid precursor proteins are constituents of the presynaptic active zone. J Neurochem 127(1):48–56
Tomita S, Kirino Y, Suzuki T (1998) Cleavage of Alzheimer’s amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism. J Biol Chem 273(11):6277–6284
Placido A et al (2014) The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis 1842(9):1444–1453
Skovronsky DM et al (2000) Protein kinase C-dependent α-secretase competes with β-secretase for cleavage of amyloid-β precursor protein in the trans-Golgi network. J Biol Chem 275(4):2568–2575
Vassar R et al (1999) β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286(5440):735–741
Huse JT et al (2000) Maturation and endosomal targeting of β-site amyloid precursor protein-cleaving enzyme the Alzheimer’s disease β-secretase. J Biol Chem 275(43):33729–33737
Pastorino L et al (2002) The carboxyl-terminus of BACE contains a sorting signal that regulates BACE trafficking but not the formation of total Aβ. Mol Cell Neurosci 19(2):175–185
Qing H et al (2004) Degradation of BACE by the ubiquitin-proteasome pathway. FASEB J 18(13):1571–1573
Zhang M et al (2012) Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem 120(6):1129–1138
Haass C et al (1992) Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 357(6378):500
Chyung JH, Raper DM, Selkoe DJ (2005) γ-Secretase exists on the plasma membrane as an intact complex that accepts substrates and effects intramembrane cleavage. J Biol Chem 280(6):4383–4392
Koo EH et al (1990) Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci 87(4):1561–1565
Araki W et al (1991) Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun 181(1):265–271
Mattson MP et al (1993) Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron 10(2):243–254
Goodman Y, Mattson MP (1994) Secreted forms of β-amyloid precursor protein protect hippocampal neurons against amyloid β-peptide-induced oxidative injury. Exp Neurol 128(1):1–12
Caillé I et al (2004) Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131(9):2173–2181
Young-Pearse TL et al (2008) Secreted APP regulates the function of full-length APP in neurite outgrowth through interaction with integrin beta1. Neural Dev 3(1):15
Thornton E et al (2006) Soluble amyloid precursor protein α reduces neuronal injury and improves functional outcome following diffuse traumatic brain injury in rats. Brain Res 1094(1):38–46
Corrigan F et al (2012) Evaluation of the effects of treatment with sAPPα on functional and histological outcome following controlled cortical impact injury in mice. Neurosci Lett 515(1):50–54
Dorard E et al (2018) Soluble amyloid precursor protein alpha interacts with alpha3-Na, K-ATPAse to induce axonal outgrowth but not neuroprotection: evidence for distinct mechanisms underlying these properties. Mol Neurobiol 55(7):5594–5610
Obregon D et al (2012) Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nat Commun 3:777
Deng J et al (2015) Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3beta signaling pathway. J Neurochem 135(3):630–637
Gakhar-Koppole N et al (2008) Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur J Neurosci 28(5):871–882
Greenberg SM, Kosik KS (1995) Secreted beta-APP stimulates MAP kinase and phosphorylation of tau in neurons. Neurobiol Aging 16(3):403–407 (discussion 407-8)
Cheng G et al (2002) Phosphatidylinositol-3-kinase-Akt kinase and p42/p44 mitogen-activated protein kinases mediate neurotrophic and excitoprotective actions of a secreted form of amyloid precursor protein. Exp Neurol 175(2):407–414
Hasebe N et al (2013) Soluble beta-amyloid precursor protein alpha binds to p75 neurotrophin receptor to promote neurite outgrowth. PLoS ONE 8(12):e82321
Tan VTY et al (2018) Lentivirus-mediated expression of human secreted amyloid precursor protein-alpha prevents development of memory and plasticity deficits in a mouse model of Alzheimer’s disease. Mol Brain 11(1):7
Luo L, Tully T, White K (1992) Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron 9(4):595–605
Torroja L et al (1999) The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci 19(18):7793–7803
Leyssen M et al (2005) Amyloid precursor protein promotes post-developmental neurite arborization in the Drosophila brain. EMBO J 24(16):2944–2955
Hornsten A et al (2007) APL-1, a Caenorhabditis elegans protein related to the human beta-amyloid precursor protein, is essential for viability. Proc Natl Acad Sci U S A 104(6):1971–1976
Wiese M, Antebi A, Zheng H (2010) Intracellular trafficking and synaptic function of APL-1 in Caenorhabditis elegans. PLoS ONE 5(9):e12790
Young-Pearse TL et al (2007) A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference. J Neurosci 27(52):14459–14469
Young-Pearse TL et al (2010) Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development. J Neurosci 30(31):10431–10440
Priller C et al (2006) Synapse formation and function is modulated by the amyloid precursor protein. J Neurosci 26(27):7212–7221
Loffler J, Huber G (1992) Beta-amyloid precursor protein isoforms in various rat brain regions and during brain development. J Neurochem 59(4):1316–1324
Corbett NJ, Hooper NM (2018) Soluble amyloid precursor protein alpha: friend or foe? Adv Exp Med Biol 1112:177–183
Pasciuto E et al (2015) Dysregulated ADAM10-mediated processing of APP during a critical time window leads to synaptic deficits in fragile X syndrome. Neuron 87(2):382–398
Westmark CJ, Malter JS (2007) FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol 5(3):e52
Furukawa K, Mattson MP (1998) The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem 70(5):1876–1886
Anderson JJ et al (1999) Reduced cerebrospinal fluid levels of alpha-secretase-cleaved amyloid precursor protein in aged rats: correlation with spatial memory deficits. Neuroscience 93(4):1409–1420
Lannfelt L et al (1995) Decreased alpha-secretase-cleaved amyloid precursor protein as a diagnostic marker for Alzheimer’s disease. Nat Med 1(8):829–832
Dobrowolska JA et al (2014) Diurnal patterns of soluble amyloid precursor protein metabolites in the human central nervous system. PLoS ONE 9(3):e89998
Paton JA, Nottebohm FN (1984) Neurons generated in the adult brain are recruited into functional circuits. Science 225(4666):1046–1048
Mu Y, Lee SW, Gage FH (2010) Signaling in adult neurogenesis. Curr Opin Neurobiol 20(4):416–423
Gould E (2007) How widespread is adult neurogenesis in mammals? Nat Rev Neurosci 8(6):481–488
Wang X et al (2016) Traumatic brain injury severity affects neurogenesis in adult mouse hippocampus. J Neurotrauma 33(8):721–733
Stolp HB, Molnar Z (2015) Neurogenic niches in the brain: help and hindrance of the barrier systems. Front Neurosci 9:20
Lim DA, Alvarez-Buylla A (2016) The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb Perspect Biol 8(5):a018820
Ming GL, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70(4):687–702
Leventhal C et al (1999) Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci 13(6):450–464
Kojima T et al (2010) Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 28(3):545–554
Mirzadeh Z et al (2008) Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3(3):265–278
Seri B et al (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21(18):7153–7160
Bond AM, Ming GL, Song H (2015) Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 17(4):385–395
Sun GJ et al (2015) Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc Natl Acad Sci U S A 112(30):9484–9489
Shen Q et al (2008) Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3(3):289–300
Delgado AC et al (2014) Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83(3):572–585
Tavazoie M et al (2008) A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3(3):279–288
Lee SW, Clemenson GD, Gage FH (2012) New neurons in an aged brain. Behav Brain Res 227(2):497–507
Richardson PM (1994) Ciliary neurotrophic factor: a review. Pharmacol Ther 63(2):187–198
Oliveira SL et al (2013) Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytometry A 83(1):76–89
Auld DS, Mennicken F, Quirion R (2001) Nerve growth factor rapidly induces prolonged acetylcholine release from cultured basal forebrain neurons: differentiation between neuromodulatory and neurotrophic influences. J Neurosci 21(10):3375–3382
Weissmiller AM, Wu C (2012) Current advances in using neurotrophic factors to treat neurodegenerative disorders. Transl Neurodegener 1(1):14
Aberg MA et al (2000) Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 20(8):2896–2903
Angelastro JM et al (2003) Regulated expression of ATF5 is required for the progression of neural progenitor cells to neurons. J Neurosci 23(11):4590–4600
Jaworski DM, Perez-Martinez L (2006) Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression is regulated by multiple neural differentiation signals. J Neurochem 98(1):234–247
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci 361(1473):1545–1564
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12):1116–1127
Mogi M et al (1999) Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett 270(1):45–48
Calissano P, Matrone C, Amadoro G (2010) Nerve growth factor as a paradigm of neurotrophins related to Alzheimer’s disease. Dev Neurobiol 70(5):372–383
Cooper JD, Lindholm D, Sofroniew MV (1994) Reduced transport of [125I]nerve growth factor by cholinergic neurons and down-regulated TrkA expression in the medial septum of aged rats. Neuroscience 62(3):625–629
Frielingsdorf H et al (2007) Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiol Dis 26(1):47–55
Pinnock SB, Herbert J (2008) Brain-derived neurotropic factor and neurogenesis in the adult rat dentate gyrus: interactions with corticosterone. Eur J Neurosci 27(10):2493–2500
Birch AM, Kelly AM (2013) Chronic intracerebroventricular infusion of nerve growth factor improves recognition memory in the rat. Neuropharmacology 75:255–261
Lu H et al (2008) Retrovirus delivered neurotrophin-3 promotes survival, proliferation and neuronal differentiation of human fetal neural stem cells in vitro. Brain Res Bull 77(4):158–164
Kamei N et al (2007) BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine (Phila Pa 1976) 32(12):1272–1278
Mu Y, Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6:85
Chuang TT (2010) Neurogenesis in mouse models of Alzheimer’s disease. Biochim Biophys Acta 1802(10):872–880
Lazarov O, Marr RA (2010) Neurogenesis and Alzheimer’s disease: at the crossroads. Exp Neurol 223(2):267–281
Winner B, Kohl Z, Gage FH (2011) Neurodegenerative disease and adult neurogenesis. Eur J Neurosci 33(6):1139–1151
Demars MP et al (2013) Soluble amyloid precursor protein-alpha rescues age-linked decline in neural progenitor cell proliferation. Neurobiol Aging 34(10):2431–2440
Mattson MP (1997) Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev 77(4):1081–1132
Rossjohn J et al (1999) Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Biol 6(4):327–331
Han P et al (2005) Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation. J Neurosci 25(50):11542–11552
Furukawa K et al (1996) Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature 379(6560):74–78
Richter MC et al (2018) Distinct in vivo roles of secreted APP ectodomain variants APPsalpha and APPsbeta in regulation of spine density, synaptic plasticity, and cognition. EMBO J 37(11):e98335
Hayashi Y et al (1994) Alzheimer amyloid protein precursor enhances proliferation of neural stem cells from fetal rat brain. Biochem Biophys Res Commun 205(1):936–943
Ohsawa I et al (1999) Amino-terminal region of secreted form of amyloid precursor protein stimulates proliferation of neural stem cells. Eur J Neurosci 11(6):1907–1913
Kwak YD et al (2006) Amyloid precursor protein regulates differentiation of human neural stem cells. Stem Cells Dev 15(3):381–389
Demars MP et al (2011) Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res Ther 2(4):36
Rohe M et al (2008) Sortilin-related receptor with A-type repeats (SORLA) affects the amyloid precursor protein-dependent stimulation of ERK signaling and adult neurogenesis. J Biol Chem 283(21):14826–14834
Baratchi S et al (2012) Secreted amyloid precursor proteins promote proliferation and glial differentiation of adult hippocampal neural progenitor cells. Hippocampus 22(7):1517–1527
Katakowski M et al (2007) Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J Cereb Blood Flow Metab 27(4):669–678
Sato Y et al (2017) Soluble APP functions as a vascular niche signal that controls adult neural stem cell number. Development 144(15):2730–2736
Donovan MH et al (2006) Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer’s disease. J Comp Neurol 495(1):70–83
Dong H et al (2004) Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 127(3):601–609
Haughey NJ et al (2002) Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem 83(6):1509–1524
Ermini FV et al (2008) Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am J Pathol 172(6):1520–1528
Cochet M et al (2013) 5-HT4 receptors constitutively promote the non-amyloidogenic pathway of APP cleavage and interact with ADAM10. ACS Chem Neurosci 4(1):130–140
Almkvist O et al (1997) Cerebrospinal fluid levels of α-secretase—cleaved soluble amyloid precursor protein mirror cognition in a Swedish family with Alzheimer disease and a gene mutation. Arch Neurol 54(5):641–644
Roch J-M et al (1994) Increase of synaptic density and memory retention by a peptide representing the trophic domain of the amyloid beta/A4 protein precursor. Proc Natl Acad Sci 91(16):7450–7454
Li T et al (2009) In-vitro effects of brain-derived neurotrophic factor on neural progenitor/stem cells from rat hippocampus. NeuroReport 20(3):295–300
Scharfman H et al (2005) Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol 192(2):348–356
Korte M et al (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci 92(19):8856–8860
Lu B et al (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14(6):401
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5(6):311
Rossjohn J et al (1999) Crystal structure of the N-terminal, growth factor-like domain of Alzheimer amyloid precursor protein. Nat Struct Mol Biol 6(4):327
Reichardt LF (2006) Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci 361(1473):1545–1564
Nakata H, Nakamura S (2007) Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett 581(10):2047–2054
Waterhouse EG et al (2012) BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J Neurosci 32(41):14318–14330
Xia D-Y et al (2017) PGC-1α or FNDC5 is involved in modulating the effects of Aβ1−42 oligomers on suppressing the expression of BDNF, a beneficial factor for inhibiting neuronal apoptosis, Aβ deposition and cognitive decline of APP/PS1 Tg mice. Front Aging Neurosci 9:65
Nagahara AH et al (2013) Early BDNF treatment ameliorates cell loss in the entorhinal cortex of APP transgenic mice. J Neurosci 33(39):15596–15602
Ohsawa I, Takamura C, Kohsaka S (1997) The amino-terminal region of amyloid precursor protein is responsible for neurite outgrowth in rat neocortical explant culture. Biochem Biophys Res Commun 236(1):59–65
Habtemariam S (2018) The brain-derived neurotrophic factor in neuronal plasticity and neuroregeneration: new pharmacological concepts for old and new drugs. Neural Regen Res 13(6):983
Chan JP et al (2008) Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci 39(3):372
Gao X, Smith GM, Chen J (2009) Impaired dendritic development and synaptic formation of postnatal-born dentate gyrus granular neurons in the absence of brain-derived neurotrophic factor signaling. Exp Neurol 215(1):178–190
Wang L et al (2015) Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J Neurosci 35(22):8384–8393
Kwak Y-D et al (2011) Involvement of notch signaling pathway in amyloid precursor protein induced glial differentiation. Eur J Pharmacol 650(1):18–27
Bell KF et al (2008) ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging 29(4):554–565
Bruno MA et al (2009) Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol 68(12):1309–1318
Mondal AC, Fatima M (2019) Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. Int J Neurosci 129(3):283–296
Zhang X et al (2014) IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway. PLoS ONE 9(12):e113801
Åberg MA et al (2003) IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci 24(1):23–40
Mir S et al (2017) IGF-1 mediated neurogenesis involves a novel RIT1/Akt/Sox2 cascade. Sci Rep 7(1):3283
Odaka H et al (2016) Chronic glucocorticoid exposure suppressed the differentiation and survival of embryonic neural stem/progenitor cells: possible involvement of ERK and PI3K/Akt signaling in the neuronal differentiation. Neurosci Res 113:28–36
O’Kusky J, Ye P (2012) Neurodevelopmental effects of insulin-like growth factor signaling. Front Neuroendocrinol 33(3):230–251
Mathews LS et al (1988) Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology 123(6):2827–2833
Cheng CM et al (2003) Insulin-like growth factor 1 is essential for normal dendritic growth. J Neurosci Res 73(1):1–9
Lin LF et al (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132
Pascual A et al (2008) Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci 11(7):755
Pascual A et al (2011) GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol 46(3):R83–R92
Kopra JJ et al (2017) Dampened amphetamine-stimulated behavior and altered dopamine transporter function in the absence of brain GDNF. J Neurosci 37(6):1581–1590
Grondin R et al (2019) GDNF revisited: a novel mammalian cell-derived variant form of GDNF increases dopamine turnover and improves brain biodistribution. Neuropharmacology 147:28–36
Boku S et al (2013) GDNF facilitates differentiation of the adult dentate gyrus-derived neural precursor cells into astrocytes via STAT3. Biochem Biophys Res Commun 434(4):779–784
Tackenberg C, Nitsch RM (2019) The secreted APP ectodomain sAPPα, but not sAPPβ, protects neurons against Aβ oligomer-induced dendritic spine loss and increased tau phosphorylation. Mol Brain 12(1):27
Rice HC et al (2019) Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission. Science 363(6423):eaao4827
Acknowledgements
The research is currently supported by grants to GWG from St. Boniface Hospital Research Foundation (SBHF-7069), Winnipeg, Canada. We would like to thank Dr. JA Bhat (University of Rochester, NY) for his constant advice throughout manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dar, N.J., Glazner, G.W. Deciphering the neuroprotective and neurogenic potential of soluble amyloid precursor protein alpha (sAPPα). Cell. Mol. Life Sci. 77, 2315–2330 (2020). https://doi.org/10.1007/s00018-019-03404-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03404-x