Abstract
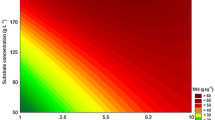
In this study, we investigated the effect of ultrasound (US) on the activity of commercial cellulase (Celluclast® 1.5 L) in the absence and in the presence of a cellulosic substrate (Avicel®, 2% w.v−1) using a central composite rotatable design. Sonication time (10 to 330 s), US intensity (120.6 to 263.7 W cm−2), and reaction temperature (25 to 50 °C) were varied using a horn-type ultrasound reactor, and endoglucanase (CMCase) and total cellulase (FPase) activities were determined. US intensity had a positive effect on enzyme activity. Under optimal conditions (170 s, 180.8 W cm−2, and 25 °C), CMCase activity was 13% higher than that of the control. In the presence of substrate, CMCase activity increased by 33.87% and KM reduced by 23% in relation to that of the control. The theoretical yield of cellulose was 42.08%. Cellulase activity can be improved by US treatment to maximize productivity gains and reduce costs in second-generation ethanol production, by the action of a low-frequency ultrasound with a short ultrasonication time of application.




Similar content being viewed by others
References
Chen, H., & Fu, X. (2016). Industrial technologies for bioethanol production from lignocellulosic biomass. Renewable and Sustainable Energy Reviews, 57, 468–478. https://doi.org/10.1016/j.rser.2015.12.069.
Soccol, C. R., Vandenberghe, L. P. de S., Medeiros, A. B. P., Karp, S. G., Buckeridge, M., Ramos, L. P., … Torres, F. A. G. (2010). Bioethanol from lignocelluloses: status and perspectives in Brazil. Bioresource Technology, 101(13), 4820–4825. doi:https://doi.org/10.1016/j.biortech.2009.11.067
Wang, Z., Lin, X., Li, P., Zhang, J., Wang, S., & Ma, H. (2012). Effects of low intensity ultrasound on cellulase pretreatment. Bioresource Technology, 117, 222–227. https://doi.org/10.1016/j.biortech.2012.04.015.
Valdivia, M., Galan, J. L., Laffarga, J., & Ramos, J. L. (2016). Biofuels 2020: biorefineries based on lignocellulosic materials. Microbial Biotechnology, 9(5), 585–594. https://doi.org/10.1111/1751-7915.12387.
Kuhad, R. C., Deswal, D., Sharma, S., Bhattacharya, A., Jain, K. K., Kaur, A., Pletschke, B. I., Singh, A., & Karp, M. (2016). Revisiting cellulase production and redefining current strategies based on major challenges. Renewable and Sustainable Energy Reviews, 55, 249–272. https://doi.org/10.1016/j.rser.2015.10.132.
Subhedar, P. B., & Gogate, P. R. (2014). Enhancing the activity of cellulase enzyme using ultrasonic irradiations. Journal of Molecular Catalysis B: Enzymatic, 101, 108–114. https://doi.org/10.1016/j.molcatb.2014.01.002.
Nguyen, T. T. T., & Le, V. V. M. (2013). Effects of ultrasound on cellulolytic activity of cellulase complex. International Food Research Journal, 20(2), 557–563.
Leaes, E. X., Lima, D., Miklasevicius, L., Ramon, A. P., Dal Prá, V., Bassaco, M. M., Terra, L. M., & Mazutti, M. A. (2013). Effect of ultrasound-assisted irradiation on the activities of α-amylase and amyloglucosidase. Biocatalysis and Agricultural Biotechnology, 2(1), 21–25. https://doi.org/10.1016/j.bcab.2012.08.003.
Dalagnol, L. M. G., Silveira, V. C. C., da Silva, H. B., Manfroi, V., & Rodrigues, R. C. (2017). Improvement of pectinase, xylanase and cellulase activities by ultrasound: effects on enzymes and substrates, kinetics and thermodynamic parameters. Process Biochemistry, 61(July), 80–87. https://doi.org/10.1016/j.procbio.2017.06.029.
Benazzi, T., Calgaroto, S., Astolfi, V., Dalla Rosa, C., Oliveira, J. V., & Mazutti, M. A. (2013). Pretreatment of sugarcane bagasse using supercritical carbon dioxide combined with ultrasound to improve the enzymatic hydrolysis. Enzyme and Microbial Technology, 52(4), 247–250. https://doi.org/10.1016/j.enzmictec.2013.02.001.
Sulaiman, A. Z., Ajit, A., Yunus, R. M., & Chisti, Y. (2011). Ultrasound-assisted fermentation enhances bioethanol productivity. Biochemical Engineering Journal, 54(3), 141–150. https://doi.org/10.1016/j.bej.2011.01.006.
Dai, C., Xiong, F., He, R., Zhang, W., & Ma, H. (2017). Effects of low-intensity ultrasound on the growth, cell membrane permeability and ethanol tolerance of Saccharomyces cerevisiae. Ultrasonics Sonochemistry, 36, 191–197. https://doi.org/10.1016/j.ultsonch.2016.11.035.
Delgado-Povedano, M. M., & Luque de Castro, M. D. (2015). A review on enzyme and ultrasound: a controversial but fruitful relationship. Analytica Chimica Acta, 889, 1–21. https://doi.org/10.1016/j.aca.2015.05.004.
Rico-Rodríguez, F., Serrato, J. C., Montilla, A., & Villamiel, M. (2018). Impact of ultrasound on galactooligosaccharides and gluconic acid production throughout a multienzymatic system. Ultrasonics Sonochemistry, 44, 177–183. https://doi.org/10.1016/J.ULTSONCH.2018.02.022.
Avhad, D. N., & Rathod, V. K. (2015). Ultrasound assisted production of a fibrinolytic enzyme in a bioreactor. Ultrasonics Sonochemistry, 22, 257–264. https://doi.org/10.1016/J.ULTSONCH.2014.04.020.
Feng, H., Barbosa-Cánovas, G. V., & Weiss, J. (2011). Ultrasound Technologies for Food and Bioprocessing. Berlin: Springer. https://doi.org/10.1007/978-1-4419-7472-3.
Szabo, O. E., Csiszar, E., Toth, K., Szakacs, G., & Koczka, B. (2015). Ultrasound-assisted extraction and characterization of hydrolytic and oxidative enzymes produced by solid state fermentation. Ultrasonics Sonochemistry, 22, 249–256. https://doi.org/10.1016/J.ULTSONCH.2014.07.001.
Huang, G., Chen, S., Dai, C., Sun, L., Sun, W., Tang, Y., Xiong, F., He, R., & Ma, H. (2017). Effects of ultrasound on microbial growth and enzyme activity. Ultrasonics Sonochemistry, 37, 144–149. https://doi.org/10.1016/j.ultsonch.2016.12.018.
Ojha, K. S., Mason, T. J., O’Donnell, C. P., Kerry, J. P., & Tiwari, B. K. (2017). Ultrasound technology for food fermentation applications. Ultrasonics Sonochemistry, 34, 410–417. https://doi.org/10.1016/J.ULTSONCH.2016.06.001.
Chemat, F., Zill-E-Huma, & Khan, M. K. (2011). Applications of ultrasound in food technology: processing, preservation and extraction. Ultrasonics Sonochemistry, 18(4), 813–835. https://doi.org/10.1016/j.ultsonch.2010.11.023.
Rokhina, E. V., Lens, P., & Virkutyte, J. (2009). Low-frequency ultrasound in biotechnology: state of the art. Trends in Biotechnology, 27(5), 298–306. https://doi.org/10.1016/j.tibtech.2009.02.001.
Szabó, O. E., & Csiszár, E. (2013). The effect of low-frequency ultrasound on the activity and efficiency of a commercial cellulase enzyme. Carbohydrate Polymers, 98(2), 1483–1489. https://doi.org/10.1016/j.carbpol.2013.08.017.
Gogate, P. R., & Kabadi, A. M. (2009). A review of applications of cavitation in biochemical engineering/biotechnology. Biochemical Engineering Journal, 44(1), 60–72. https://doi.org/10.1016/j.bej.2008.10.006.
Szabo, O. E., & Csiszar, E. (2017). Some factors affecting efficiency of the ultrasound-aided enzymatic hydrolysis of cotton cellulose. Carbohydrate Polymers, 156, 357–363. https://doi.org/10.1016/j.carbpol.2016.09.039.
Islam, M. N., Zhang, M., & Adhikari, B. (2014). The inactivation of enzymes by ultrasound-a review of potential mechanisms. Food Reviews International, 30(1), 1–21. https://doi.org/10.1080/87559129.2013.853772.
Rodrigues, M. I., & Iemma, A. F. (2014). Design of Experiments and Process Optimization (1st ed.). Campinas: CRC Press.
Humbird, D., Davis, R., Tao, L., Kinchin, C., Hsu, D., Aden, A., et al. (2011). Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. Renewable Energy, 303(May), 147. https://doi.org/10.2172/1013269.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and Applied Chemistry, 59(2), 257–268. https://doi.org/10.1351/pac198759020257.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. https://doi.org/10.1021/ac60147a030.
Qiu, Z., Aita, G. M., & Walker, M. S. (2012). Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresource Technology, 117, 251–256. https://doi.org/10.1016/j.biortech.2012.04.070.
Nadar, S. S., & Rathod, V. K. (2017). Sonochemical effect on activity and conformation of commercial lipases. Applied Biochemistry and Biotechnology, 181(4), 1435–1453. https://doi.org/10.1007/s12010-016-2294-2.
Ma, H., Huang, L., Jia, J., He, R., Luo, L., & Zhu, W. (2011). Effect of energy-gathered ultrasound on Alcalase. Ultrasonics Sonochemistry, 18(1), 419–424. https://doi.org/10.1016/J.ULTSONCH.2010.07.014.
Guiseppi-Elie, A., Choi, S.-H., & Geckeler, K. E. (2009). Ultrasonic processing of enzymes: effect on enzymatic activity of glucose oxidase. Journal of Molecular Catalysis B: Enzymatic, 58(1), 118–123. https://doi.org/10.1016/j.molcatb.2008.12.005.
Singh, S., Bharadwaja, S. T. P., Yadav, P. K., Moholkar, V. S., & Goyal, A. (2014). Mechanistic investigation in ultrasound-assisted (alkaline) delignification of parthenium hysterophorus biomass. Industrial and Engineering Chemistry Research, 53(37), 14241–14252. https://doi.org/10.1021/ie502339q.
Oliveira, H. M., Correia, V. S., Segundo, M. A., Fonseca, A. J. M., & Cabrita, A. R. J. (2017). Does ultrasound improve the activity of alpha amylase? A comparative study towards a tailor-made enzymatic hydrolysis of starch. LWT - Food Science and Technology, 84, 674–685. https://doi.org/10.1016/j.lwt.2017.06.035.
Yu, Z. L., Zeng, W. C., Zhang, W. H., Liao, X. P., & Shi, B. (2014). Effect of ultrasound on the activity and conformation of α-amylase, papain and pepsin. Ultrasonics Sonochemistry, 21(3), 930–936. https://doi.org/10.1016/j.ultsonch.2013.11.002.
Aliyu, M., & Hepher, M. J. (2000). Effects of ultrasound energy on degradation of cellulose material. Ultrasonics Sonochemistry, 7(4), 265–268. https://doi.org/10.1016/S1350-4177(00)00052-3.
Yachmenev, V. G., Bertoniere, N. R., & Blanchard, E. J. (2002). Intensification of the bio-processing of cotton textiles by combined enzyme/ultrasound treatment. Journal of Chemical Technology and Biotechnology, 77(5), 559–567. https://doi.org/10.1002/jctb.579.
Sulaiman, A. Z., & Ajit, A. (2013). Ultrasound mediated enzymatic hydrolysis of cellulose and carboxymethyl cellulose. https://doi.org/10.1002/btpr.1786.
Funding
This study was supported by CNPq (grant no. 444422/2014-5), FAPESP (grant nos. 04602-3/2016 and 11932-7/2015), and CAPES (finance code 001). We also thank CAPES for the scholarship provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Carvalho Silvello, M.A., Martínez, J. & Goldbeck, R. Low-frequency Ultrasound with Short Application Time Improves Cellulase Activity and Reducing Sugars Release. Appl Biochem Biotechnol 191, 1042–1055 (2020). https://doi.org/10.1007/s12010-019-03148-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03148-1