Abstract
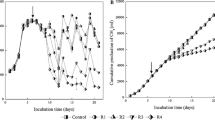
Isovalerate is one of the key intermediates during anaerobic digestion treating protein-containing waste/wastewater. Investigating the effect of different kinds of inhibitors on isovalerate-degrading microbial community is necessary to develop measures for improving the effectiveness of the treatment plants. In the present study, dynamic changes in the isovalerate-degrading microbial community in presence of inhibitors (ammonium, sulfide, mixed ammonium and sulfide, and chlortetracycline (CTC)) were investigated using high-throughput sequencing of 16S rRNA gene. Our observations showed that the isovalerate-degrading microbial community responded differently to different inhibitors and that the isovalerate degradation and gas production were strongly repressed by each inhibitor. We found that sulfide inhibited both isovalerate oxidation followed by methanogenesis, while ammonium, mixed ammonium and sulfide, and CTC mainly inhibited isovalerate oxidation. Genera classified into Proteobacteria and Chloroflexi were less sensitive to inhibitors. The two dominant genera, which are potential syntrophic isovalerate oxidizers, exhibited different responses to inhibitors that the unclassified_Peptococcaceae_3 was more sensitive to inhibitors than the unclassified_Syntrophaceae. Upon comparison to acetoclastic methanogen Methanosaeta, hydrogenotrophic methanogens Methanoculleus and Methanobacterium were less sensitive to inhibitors.




Similar content being viewed by others
References
Tian, H., Karachalios, P., Angelidaki, I., & Fotidis, I. A. (2018). A proposed mechanism for the ammonia–LCFA synergetic co-inhibition effect on anaerobic digestion process. Chemical Engineering Journal, 349, 574–580.
Kamali, M., Gameiro, T., Costa, M. E. V., & Capela, I. (2016). Anaerobic digestion of pulp and paper mill wastes—an overview of the developments and improvement opportunities. Chemical Engineering Journal, 298, 162–182.
Zhao, L., Dong, Y. H., & Wang, H. (2010). Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. The Science of the Total Environment, 408(5), 1069–1075.
Wang, H. Z., Yan, Y. C., Gou, M., Yi, Y., Xia, Z. Y., Nobu, M. K., Narihiro, T., & Tang, Y. Q. (2019). Response of propionate-degrading methanogenic microbial communities to inhibitory conditions. Applied Biochemistry and Biotechnology, 189(1), 233–248.
Zhang, C., Yuan, Q., & Lu, Y. (2018). Inhibitory effects of ammonia on syntrophic propionate oxidation in anaerobic digester sludge. Water Research, 146, 275–287.
Yang, Z., Wang, W., He, Y., Zhang, R., & Liu, G. (2018). Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions. Renewable Energy, 125, 915–925.
Lü, F., Hao, L., Guan, D., Qi, Y., Shao, L., & He, P. (2013). Synergetic stress of acids and ammonium on the shift in the methanogenic pathways during thermophilic anaerobic digestion of organics. Water Research, 47(7), 2297–2306.
Karakashev, D., Batstone, D. J., & Angelidaki, I. (2005). Influence of environmental conditions on methanogenic compositions in anaerobic biogas reactors. Applied and Environmental Microbiology, 71(1), 331–338.
Koster, I. W., & Lettinga, G. (1984). The influence of ammonium-nitrogen on the specific activity of pelletized methanogenic sludge. Agricultural Wastes, 9, 205–216.
Westerholm, M., Levén, L., & Schnürer, A. (2012). Bioaugmentation of syntrophic acetate-oxidizing culture in biogas reactors exposed to increasing levels of ammonia. Applied and Environmental Microbiology, 78(21), 7619–7625.
Angelidaki, I., & Ahring, B. K. (1993). Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Applied Microbiology and Biotechnology, 38, 560–564.
Chen, S., He, J., Wang, H., Dong, B., Li, N., & Dai, X. (2018). Microbial responses and metabolic pathways reveal the recovery mechanism of an anaerobic digestion system subjected to progressive inhibition by ammonia. Chemical Engineering Journal, 350, 312–323.
Turker, G., Aydin, S., Akyol, Ç., Yenigun, O., Ince, O., & Ince, B. (2016). Changes in microbial community structures due to varying operational conditions in the anaerobic digestion of oxytetracycline-medicated cow manure. Applied Microbiology and Biotechnology, 100(14), 6469–6479.
Deng, Y., Zhang, Y., Gao, Y., Li, D., Liu, R., Liu, M., Zhang, H., Hu, B., Yu, T., & Yang, M. (2012). Microbial community compositional analysis for series reactors treating high level antibiotic wastewater. Environmental Science & Technology, 46(2), 795–801.
Pind, P. F., Angelidaki, I., & Ahring, B. K. (2003). Dynamics of the anaerobic process: effects of volatile fatty acids. Biotechnology and Bioengineering, 82(7), 791–801.
Müller, N., Worm, P., Schink, B., Stams, A. J. M., & Plugge, C. M. (2010). Syntrophic butyrate and propionate oxidation processes: from genomes to reaction mechanisms. Environmental Microbiology Reports, 2(4), 489–499.
Amani, T., Nosrati, M., Mousavi, S. M., & Kermanshahi, R. K. (2011). Study of syntrophic anaerobic digestion of volatile fatty acids using enriched cultures at mesophilic conditions. International journal of Environmental Science and Technology, 8, 83–96.
Batstone, D. J., Pind, P. F., & Angelidaki, I. (2003). Kinetics of thermophilic, anaerobic oxidation of straight and branched chain butyrate and valerate. Biotechnology and Bioengineering, 84(2), 195–204.
Stieb, M., & Schink, B. (1986). Anaerobic degradation of isovalerate by a defined methanogenic coculture. Archives of Microbiology, 144, 291–295.
Narihiro, T., Nobu, M. K., Tamaki, H., Kamagata, Y., Sekiguchi, Y., & Liu, W. T. (2016). Comparative genomics of syntrophic branched-chain fatty acid degrading bacteria. Microbes and Environments, 31(3), 288–292.
Nobu, M. K., Narihiro, T., Rinke, C., Kamagata, Y., Tringe, S. G., Woyke, T., & Liu, W. T. (2015). Microbial dark matter ecogenomics reveals complex synergistic networks in a methanogenic bioreactor. The ISME Journal, 9(8), 1710–1722.
Shigematsu, T., Tang, Y. Q., Kawaguchi, H., Ninomiya, K., Kijima, J., Kobayashi, T., Morimura, S., & Kida, K. (2003). Effect of dilution rate on structure of a mesophilic acetate-degrading methanogenic community during continuous cultivation. Journal of Bioscience and Bioengineering, 96(6), 547–558.
Jiang, X., Hayashi, J., Sun, Z. Y., Yang, L., Tang, Y. Q., Oshibe, H., Osaka, N., & Kida, K. (2013). Improving biogas production from protein-rich distillery wastewater by decreasing ammonia inhibition. Process Biochemistry, 48, 1778–1784.
Griffiths, R. I., Whiteley, A. S., Donnell, A. G., & Bailey, M. J. (2000). Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Applied and Environmental Microbiology, 66(12), 5488–5491.
Dan, X., Chen, H., Chen, F., He, Y., Zhao, C., Zhu, D., Zeng, L., & Li, W. (2016). Analysis of the rumen bacteria and methanogenic archaea of yak (Bos grunniens) steers grazing on the Qinghai–Tibetan plateau. Livestock Science, 188, 61–71.
Zheng, D., Wang, H. Z., Gou, M., Nobu, M. K., Narihiro, T., Hu, B., Nie, Y., & Tang, Y. Q. (2019). Identification of novel potential acetate-oxidizing bacteria in thermophilic methanogenic chemostats by DNA stable isotope probing. Applied Microbiology and Biotechnology, 103(20), 8631–8645.
Greetham, D., Hart, A. J., & Tucker, G. A. (2016). Presence of low concentrations of acetic acid improves yeast tolerance to hydroxymethylfurfural (HMF) and furfural. Biomass and Bioenergy, 85, 53–60.
Dörries, M., Wöhlbrand, L., Kube, M., Reinhardt, R., & Rabus, R. (2016). Genome and catabolic subproteomes of the marine, nutritionally versatile, sulfate-reducing bacterium Desulfococcus multivorans DSM 2059. BMC Genomics, 17, 918.
Zheng, D., & Raskin, L. (2000). Quantification of Methanosaeta species in anaerobic bioreactors using genus- and species-specific hybridization probes. Microbial Ecology, 39(3), 246–262.
Tan, L., Cheng, Q. S., Sun, Z. Y., Tang, Y. Q., & Kida, K. (2019). Effects of ammonium and/or sulfide on methane production from acetate or propionate using biochemical methane potential tests. Journal of Bioscience and Bioengineering, 127(3), 345–352.
Torres, N. P., Lee, A. Y., Giaever, G., Nislow, C., & Brown, G. W. (2013). A high-throughput yeast assay identifies synergistic drug combinations. Assay and Drug Development Technologies, 11(5), 299–307.
Stone, J. J., Clay, S. A., Zhu, Z., Wong, K. L., Porath, L. R., & Spellman, G. M. (2009). Effect of antimicrobial compounds tylosin and chlortetracycline during batch anaerobic swine manure digestion. Water Research, 43(18), 4740–4750.
Yin, F., Dong, H., Ji, C., Tao, X., & Chen, Y. (2016). Effects of anaerobic digestion on chlortetracycline and oxytetracycline degradation efficiency for swine manure. Waste Management, 56, 540–546.
Akyol, Ç., Aydin, S., Ince, O., & Ince, B. (2016). A comprehensive microbial insight into single-stage and two-stage anaerobic digestion of oxytetracycline-medicated cattle manure. Chemical Engineering Journal, 303, 675–684.
Lu, Y., Liaquat, R., Astals, S., Jensen, P. D., Batstone, D. J., & Tait, S. (2018). Relationship between microbial community, operational factors and ammonia inhibition resilience in anaerobic digesters at low and moderate ammonia background concentrations. New Biotechnology, 44, 23–30.
Aydin, S., Ince, B., & Ince, O. (2015). Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Research, 76, 88–98.
Cheng, D. L., Ngo, H. H., Guo, W. S., Chang, S. W., Nguyen, D. D., Kumar, S. M., Du, B., Wei, Q., & Wei, D. (2018). Problematic effects of antibiotics on anaerobic treatment of swine wastewater. Bioresource Technology, 263, 642–653.
Barberán, A., Bates, S. T., Casamayor, E. O., & Fierer, N. (2011). Using network analysis to explore co-occurrence patterns in soil microbial communities. The ISME Journal, 6, 343–351.
Yoon, K. S., Tsukada, N., Sakai, Y., Ishii, M., Igarashi, Y., & Nishihara, H. (2008). Isolation and characterization of a new facultatively autotrophic hydrogen-oxidizing Betaproteobacterium, Hydrogenophaga sp. AH-24. FEMS Microbiology Letters, 278, 94–100.
Tian, Z., Zhang, Y., & Yang, M. (2018). Chronic impacts of oxytetracycline on mesophilic anaerobic digestion of excess sludge: inhibition of hydrolytic acidification and enrichment of antibiotic resistome. Environmental Pollution, 238, 1017–1026.
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (No. 2016YFE0127700) and the National Natural Science Foundation of China (No. 51678378).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This paper does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3023 kb)
Rights and permissions
About this article
Cite this article
Li, J., Wang, HZ., Yi, Y. et al. Response of Isovalerate-Degrading Methanogenic Microbial Community to Inhibitors. Appl Biochem Biotechnol 191, 1010–1026 (2020). https://doi.org/10.1007/s12010-020-03234-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03234-9