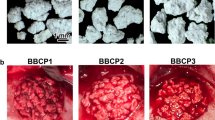
Abstract
Objectives
Biphasic calcium phosphates (BCP) are synthetic biomaterials developed as an alternative to the autogenous bone grafts and xenografts. The aim of the present study was to assess the influence of the addition of collagen onto the BCP resorption rate and bone formation.
Material and methods
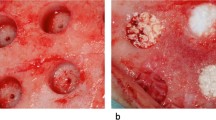
Eighteen male NWZ rabbits approximately 12 weeks of age were used. Critical size defects were randomly treated with bilayered BCP materials comprising 12% HA and 88% α-TCP with and without collagen or sham-operated, respectively. All defects were covered with a resorbable collagen membrane. Animals were euthanized after 3 and 12 weeks of healing and investigated by micro-CT, histologic, and histomorphometric analysis.
Results
Woven bone formation was observed from the original bone at 3-week healing in all samples. After 3 months, mainly lamellar new bone in the peripheral area was observed. In the central region, both woven and lamellar bone were seen. Samples containing collagen showed less residual biomaterial than without collagen at both healing periods. Both types of granules were in close contact with new bone, yielding a complete defect closure at 3 months of healing. However, new bone volume and area was similar for both biomaterials.
Conclusions
Within its limitations, the study results qualify collagen as a biocompatible carrier for BCPs. The presence of collagen indicated neither significant impact on the resorption of the BCPs nor on bone formation.
Clinical relevance
The addition of collagen to BCPs might not be beneficial for the augmentation of extended bone deficiencies.





Similar content being viewed by others
References
Miron RJ, Hedbom E, Saulacic N, Zhang Y, Sculean A, Bosshardt DD, Buser D (2011) Osteogenic potential of autogenous bone grafts harvested with four different surgical techniques. J Dent Res 90:1428–1433
Nkenke E, Stelzle F (2009) Clinical outcomes of sinus floor augmentation for implant placement using autogenous bone or bone substitutes: a systematic review. Clin Oral Implants Res 20(Suppl 4):124–133
Wang RE, Lang NP (2012) Ridge preservation after tooth extraction. Clin Oral Implants Res 23(Suppl 6):147–156
Williams RJ, Gamradt SC (2008) Articular cartilage repair using a resorbable matrix scaffold. Instr Course Lect 57:563–571
Kolerman R, Samorodnitzky-Naveh GR, Barnea E, Tal H (2012) Histomorphometric analysis of newly formed bone after bilateral maxillary sinus augmentation using two different osteoconductive materials and internal collagen membrane. Int J Periodontics Restorative Dent 32:e21–e28
Mordenfeld A, Hallman M, Johansson CB, Albrektsson T (2010) Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin Oral Implants Res 21:961–970
Hwang JW, Park JS, Lee JS, Jung UW, Kim CS, Cho KS, Lee YK, Choi SH (2012) Comparative evaluation of three calcium phosphate synthetic block bone graft materials for bone regeneration in rabbit calvaria. J biomed mater res. B Appl Biomat 100:2044–2052
Torres J, Tamimi F, Alkhraisat MH, Prados-Frutos JC, Rastikerdar E, Gbureck U, Barralet JE, Lopez-Cabarcos E (2011) Vertical bone augmentation with 3D-synthetic monetite blocks in the rabbit calvaria. J Clin Periodontol 38:1147–1153
Rojbani H, Nyan M, Ohya K, Kasugai S (2011) Evaluation of the osteoconductivity of alpha-tricalcium phosphate, beta-tricalcium phosphate, and hydroxyapatite combined with or without simvastatin in rat calvarial defect. J Biomed Mater Res A 98:488–498
Hirota M, Matsui Y, Mizuki N, Kishi T, Watanuki K, Ozawa T, Fukui T, Shoji S, Adachi M, Monden Y, Iwai T, Tohnai I (2009) Combination with allogenic bone reduces early absorption of beta-tricalcium phosphate (beta-TCP) and enhances the role as a bone regeneration scaffold. Experimental animal study in rat mandibular bone defects. Dent Mater J 28:153–161
Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K (2005) 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 26:3565–3575
Carrodeguas RG, De Aza S (2011) Alpha-tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater 7:3536–3546
Daculsi G, Laboux O, Malard O, Weiss P (2003) Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med 14:195–200
Nery EB, LeGeros RZ, Lynch KL, Lee K (1992) Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J Periodontol 63:729–735
Bouler JM, Pilet P, Gauthier O, Verron E (2017) Biphasic calcium phosphate ceramics for bone reconstruction: a review of biological response. Acta Biomater 53:1–12
Helder MN, van Esterik FAS, Kwehandjaja MD, Ten Bruggenkate CM, Klein-Nulend J, Schulten E (2018) Evaluation of a new biphasic calcium phosphate for maxillary sinus floor elevation: micro-CT and histomorphometrical analyses. Clin Oral Implants Res 29:488–498
Jensen SS, Bornstein MM, Dard M, Bosshardt DD, Buser D (2009) Comparative study of biphasic calcium phosphates with different HA/TCP ratios in mandibular bone defects A long-term histomorphometric study in minipigs. J Biomed Mater Res B App Biomater 90:171–181
Yang C, Unursaikhan O, Lee JS, Jung UW, Kim CS, Choi SH (2014) Osteoconductivity and biodegradation of synthetic bone substitutes with different tricalcium phosphate contents in rabbits. J Biomed Mater Res B App Biomater 102:80–88
Yip I, Ma L, Mattheos N, Dard M, Lang NP (2015) Defect healing with various bone substitutes. Clin Oral Implants Res 26:606–614
Carrel JP, Wiskott A, Moussa M, Rieder P, Scherrer S, Durual S (2016) A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin Oral Implants Res 27:55–62
Bohner M (2000) Calcium orthophosphates in medicine: from ceramics to calcium phosphate cements. Injury 31(Suppl 4):37–47
Sawada K, Nakahara K, Haga-Tsujimura M, Iizuka T, Fujioka-Kobayashi M, Igarashi K, Saulacic N (2018) Comparison of three block bone substitutes for bone regeneration: long-term observation in the beagle dog. Odontology 106:398–407
Lindhe J, Araujo MG, Bufler M, Liljenberg B (2013) Biphasic alloplastic graft used to preserve the dimension of the edentulous ridge: an experimental study in the dog. Clin Oral Implants Res 24:1158–1163
Wagner-Ecker M, Voltz P, Egermann M, Richter W (2013) The collagen component of biological bone graft substitutes promotes ectopic bone formation by human mesenchymal stem cells. Acta Biomater 9:7298–7307
Sheikh Z, Brooks PJ, Barzilay O, Fine N, Glogauer M (2015) Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 8:5671–5701
Miron RJ, Bosshardt DD (2016) OsteoMacs: key players around bone biomaterials. Biomaterials 82:1–19
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. Semin Immunol 20:86–100
Klenke FM, Liu Y, Yuan H, Hunziker EB, Siebenrock KA, Hofstetter W (2008) Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J Biomed Mater Res A 85:777–786
Habibovic P, Kruyt MC, Juhl MV, Clyens S, Martinetti R, Dolcini L, Theilgaard N, van Blitterswijk CA (2008) Comparative in vivo study of six hydroxyapatite-based bone graft substitutes. J Orthop Res 26:1363–1370
Park JW, Kim ES, Jang JH, Suh JY, Park KB, Hanawa T (2010) Healing of rabbit calvarial bone defects using biphasic calcium phosphate ceramics made of submicron-sized grains with a hierarchical pore structure. Clin Oral Implants Res 21:268–276
Acknowledgments
The authors wish to thank the staff at the ESI, Surgical Unit, DBMR, University of Bern, Switzerland, for excellent handling of the animals, Mr. Mark Siegrist for his assistance during μCT evaluation and Ms. Inga Grigaitiene for the histological preparation. The synthetic bone substitutes were kindly provided by Geistlich Pharma AG (Wolhusen, Switzerland).
Funding
The study was supported by the Department of Cranio-Maxillofacial Surgery, Faculty of Medicine, University of Berne, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved from the Committee for Animal Research, Canton of Berne, Switzerland (Nr: BE 89/17).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Suppl. Fig. 1
Surface appearance of the particles of BBCP and BBCP_C. The rough surfaces with pores are observable on each particle. Collagen matrix is further contained in BBCP_C material (PNG 3497 kb)
Suppl. Fig. 2
Toluidine blue and fuchsin staining of BBCP and BBCP_C particles after mixed with blood during surgery. Blood cells (yellow arrowheads) are attached and kept on the surface of bone substitutes despite a lot of liquid handling. Collagen fibers (black arrows) also encapsulated blood cells (yellow arrowheads) in BBCP_C materials (PNG 5042 kb)
Suppl. Fig. 3
Center of the defect next to the dura mater treated with BBCP (A) and the periphery of defect treated with BBCP_C granules (B) after 3 weeks of healing. Direct contact of new bone (NB) is restricted to the surface of both type of granules (*) (PNG 5176 kb)
Suppl. Fig. 4
Higher magnification of the periphery of the defects treated with BBCP (A) and BBCP_C (B) granules after 3 weeks of healing. Apposition of new bone (NB) is observed in the large pores of both types of granules (*) (PNG 4649 kb)
Suppl. Fig. 5
Center of the defects treated with BBCP (A) and BBCP_C (B) granules after 3 weeks of healing. Nonintegrated granules (*) are covered with a plenty of multinucleated giant cells (arrowheads) and a condensed fibrous tissue (PNG 5477 kb)
Suppl. Fig. 6
Periphery of the defect treated with BBCP (A) and center of the defect treated with BBCP_C granules (B) after 3 months of healing. Residual granules (*) are embedded in the newly formed bone (NB). Osteons (arrowheads) are observed within the newly formed lamellar bone and perforating the granules (PNG 5033 kb)
Suppl. Fig. 7
Higher magnification of the center of the defect treated with BBCP (A) and BBCP_C (B) granules after 3 months of healing. Residual granules (*) are almost completely surrounded by a layer of multinuclear cells (arrowheads). Cell poor and highly vascularized loose connective tissue is observed between the granules (PNG 5290 kb)
Rights and permissions
About this article
Cite this article
Schaller, B., Fujioka-Kobayashi, M., Zihlmann, C. et al. Effects of additional collagen in biphasic calcium phosphates: a study in a rabbit calvaria. Clin Oral Invest 24, 3093–3103 (2020). https://doi.org/10.1007/s00784-019-03181-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03181-8