Abstract
Six components of partial resistance (RCs) were studied in 15 grapevine varieties with partial resistance to Plasmopara viticola: (i) infection frequency (IFR, proportion of inoculation sites showing sporulation), (ii) latent period (LP50, degree-days between inoculation and appearance of 50% of the final number of sporulating lesions), (iii) lesion size (LS, area of single lesions in mm2), (iv) production of sporangia (SPOR, number of sporangia produced per lesion, and SPOR’, number of sporangia produced per mm2 of lesion), (v) infectious period (IP, number of sporulation events on a lesion), and (vi) infectivity of sporangia (INF, infection efficiency of sporangia produced on resistant varieties). Artificial inoculation monocycle experiments were conducted for a 3-year period on leaves collected at leaf development, flowering, and fruit development. Compared to the susceptible variety ‘Merlot’, the partially resistant varieties showed reduced IFR, longer LP, smaller LS, fewer SPOR and SPOR’, shorter IP, and lower INF. At leaf development, IFR, SPOR, and INF were higher and LP was shorter than at flowering and fruit development. RCs analysis through monocyclic experiments provides reliable assessments of the resistance response of grapevine accessions. The workload required for routine assessment in breeding programs could be reduced by measuring IFR and SPOR, while producing robust results.
Similar content being viewed by others
Introduction
Downy mildew (DM) of grapevine is a serious disease caused by the obligate, biotrophic Oomycete Plasmopara viticola1. P. viticola originated from North America, was introduced in Europe in the 1870 s2, and spread across the continent in the following years1.
The level of partial resistance to DM varies widely among Vitis species and cultivars3,4. The Eurasian grapevine V. vinifera, which is widely cultivated for its agronomic and quality traits, is generally susceptible to DM, whereas Asian and American Vitis species (e.g., V. riparia and V. rupestris) show varying degrees of resistance5 because of their coevolution with the pathogen. The partial resistance to P. viticola is conferred to grapevines by specific loci referred to as Rpvs (resistance to P. viticola)6; partial resistance has also been found in V. vinifera germplasm7.
Breeding programs have been implemented based on the hybridization of American Vitis spp. with V. vinifera for the introgression of resistance genes into the domesticated background of V. vinifera. Some of the breeding lines, after several cycles of backcrossing, gained more than 80% of V. vinifera genetic background8, and incorporated one or more (pyramided) Rpvs. Some reports, which characterized partially resistant varieties under vineyard conditions, indicate a reduction in the speed of DM epidemics on resistant varieties in comparison with susceptible grapevine ones9,10. Since the early 1900s, several partially resistant varieties with good grape quality and in some cases capable of producing a vinifera-like wine11,12,13 have been selected and released by breeders. The resistance response of grapevines to DM has been well investigated7,10,14,15,16,17,18,19,20,21. Resistance mechanisms include a hypersensitive response (HR), the synthesis and accumulation of polyphenols (i.e., stilbene and flavonoid phytoalexins), the production of reactive oxygen species, and callose deposition14,22,23,24.
Partial resistance may influence several stages of the infection cycle, including spore germination, penetration into the host tissue, colonization of the inner host tissue, the duration of latent and infectious periods, and sporulation25. These processes are called “components of partial resistance” and regulate the epidemic that can result from a chain of infection cycles during the host-growing season. In partial resistance, the phenotype is the result of different phases of the infection process in which resistance responses occur, and each phase is considered a component of resistance that contributes to the overall response26. The most studied components of resistance include reduced infection efficiency, a longer latent period, a shorter infectious period, reduced sporulation, and a smaller lesion size27,28,29,30. Resistance components have been analyzed31 for a number of pathosystems, including Cercospora leaf spot of sugar beet29,32, yellow rust (Puccinia striiformis f. sp. hordei) of barley33, rice blast (Pyricularia oryzae)34, rice sheath blight (Rhizoctonia solani)35, leaf rust (Puccinia triticina) of wheat36, Fusarium head blight of wheat37, and Phoma black stem of sunflower38. However, such analysis has not been conducted on the DM–grapevine pathosystem.
From an epidemiological point of view, components of partial resistance all help to reduce the apparent infection rate (r) of the epidemic, thus slowing disease progress25,39,40,41,42. The effects of partial resistance components (RCs) on the epidemic development have been investigated for groundnut rust43, potato late blight44, wheat stripe rust45, and sugar beet Cercospora leaf spot32.
The objective of this work was to measure the RCs to P. viticola in 15 Vitis accessions, most of them carry one or more Rpvs (www.vivc.de), or showing moderate susceptibility to downy mildew. For the sake of simplicity, these 15 accessions are referred to as “partially resistant varieties” in this paper. The following phenotypic traits were assessed in monocycle experiments conducted under environmentally controlled conditions: (i) degree of resistance, based on the OIV 452-1 descriptor46; (ii) infection frequency; (iii) duration of the latent period; (iv) lesion size; (v) number of sporangia produced per lesion and per lesion surface unit, (vi) duration of the infectious period; and (vii) infectivity of the sporangia produced on DM lesions.
Results
Degree of resistance in the tested varieties
A factorial analysis of variance (ANOVA) for the degree of resistance to P. viticola according to the OIV 452-1 descriptor46 showed significant effects of year, growth stage, variety, and their interactions (P < 0.001). Year and growth stage accounted for 3.7% and 3.5% of total variance, respectively, while variety accounted for 63% (Supplementary Table S1). The susceptible control ‘Merlot’ scored 1.4 in resistance to DM (very low resistance). Among the tested varieties, the lowest OIV 452-1 value was 1.6 (for ‘Rkatsitelii’), and the highest values were 6.9 (for ‘Bronner’) and 7.9 (for ‘Johanniter’) (Fig. 1).
Resistance to Plasmopara viticola assessed on leaf discs of 16 grapevine varieties (X axis) after artificial inoculation. The degree of resistance is expressed according to the descriptor OIV 452-146 : 1 = very low degree of resistance; 3 = low degree of resistance; 5 = medium degree of resistance; 7 = high degree of resistance; and 9 = very high degree of resistance. Values are means of data collected in a 3-year period (from 2014 to 2016) and at three growth stage, with 75 replicate leaf discs for each variety in each experiment + SE. Values with different letters are significantly different according to the LSD test (P = 0.05).
Resistance components in ‘Merlot’ and in the partially resistant varieties
ANOVAs showed a significant effect (P < 0.001) of variety for all RCs (Table 1). Variety and its interactions accounted for 67% to 89% of the total variance, depending on the RC (Supplementary Table S1). Plant growth stage at the time of RC measurement also had a significant effect (P ≤ 0.01) on RCs; the interaction variety × growth stage was significant for some RCs but not for others, and accounted for only a small percentage of the total variance (Supplementary Table S1). Our analysis now focuses on the main effects of variety (Table 1) and growth stage (Table 2).
The overall average values for the infection frequency (IFR) for ‘Rkatsitelii’, ‘Calandro’, ‘Cabernet Volos’, ‘Villaris’, and ‘Regent’ were not significantly different from that of the susceptible control ‘Merlot’, which had an average IFR value of 0.95 (Table 1). IFR values were lower for ‘Reberger’, ‘Felicia’, ‘Fleurtai’, ‘Palava’, ‘Merlot Khorus’, ‘Solaris’, ‘Calardis Blanc’, ‘Merlot Kanthus’, ‘Bronner’, and ‘Johanniter’ than for ‘Merlot’, with a reduction relative to ‘Merlot’ ranging from 6% for ‘Reberger’ to 43% for ‘Bronner’ and ‘Johanniter’ (Table 1). The average IFR value was significantly higher at growth stage 18, i.e. shoot growing (IFR = 0.85), than at stages 65, i.e. flowering (IFR = 0.81), or 79, i.e. fruit development (IFR = 0.82) (Table 2).
Area Under Infection Progress Curve (AUIPC) for ‘Cabernet Volos’ and ‘Rkatsitelii’ were significantly lower and higher, respectively, than the value for the susceptible control ‘Merlot’ (Table 1). AUIPC values for all of the other varieties were lower than the AUIPC for ‘Merlot’, with reductions relative to ‘Merlot’ ranging from 4% to 50%. AUIPC values were lowest for ‘Bronner’ and ‘Johanniter’. As was the case for IFR, the average AUIPC was significantly higher at stage 18 than at stages 65 or 79 (Table 2).
The duration of the latent period (LP50) did not significantly differ between ‘Villaris’ and the susceptible control ‘Merlot’ (Table 1). Relative to ‘Merlot’ and ‘Villaris’, the LP50 was shorter for ‘Rkatsitelii’ but was longer for all other varieties. The LP50 was longest for ‘Johanniter’ and ‘Bronner’, with an increase of 20% and 17%, respectively, relative to ‘Merlot’. The LP50 was significantly shorter at growth stage 18 than at the other two growth stages, which were not significantly different each other (Table 2).
The lesion size (LS) was smaller for all the partially resistant varieties than for ‘Merlot’ (Table 1), with reductions ranging from 23% to >90% (93% for ‘Calandro’ and 96% for ‘Johanniter’).
The number of sporangia produced per mm2 of DM lesion (SPOR’) was higher for ‘Reberger’, ‘Johanniter’, and ‘Calandro’ than for ‘Merlot’, and did not significantly differ among ‘Rkatsitelii’, ‘Palava’, and ‘Merlot’ (Table 1). For all other varieties, SPOR’ values were reduced by 50% to 95% compared to ‘Merlot’, and values were lowest values for ‘Bronner’, ‘Fleurtai’, and ‘Merlot Kanthus’. SPOR’ values were higher at stage 18 than at the other two stages (Table 2).
The number of sporangia produced per lesion (SPOR) on ‘Reberger’ was not significantly different from the number on ‘Merlot’ (Table 1). In the partially resistant varieties, the SPOR was reduced by 44% to 99% compared to ‘Merlot’. SPOR was lowest on ‘Bronner’, ‘Merlot Kanthus’, and ‘Fleurtai’. Like SPOR’ values, SPOR values were higher at stage 18 than at the other two stages (Table 2).
The infectious period (IP) for ‘Regent’, ‘Solaris’, ‘Felicia’, ‘Calandro’, ‘Merlot Khorus’, and ‘Reberger’ was not significantly different from that for ‘Merlot’ (Table 1), while the IP was longer for ‘Villaris’, ‘Cabernet Volos’, and ‘Fleurtai’. For ‘Palava’, ‘Johanniter’, ‘Rkatsitelii’, ‘Merlot Kanthus’, ‘Calardis Blanc’, and ‘Bronner’, the number of sporulation events was 1/3 to 2/3 lower than for the control ‘Merlot’.
For most varieties, the infectivity of sporangia produced on the DM lesions (INF) did not significantly differ from that of the sporangia produced on ‘Merlot’ (Table 1). INF, however, was significantly higher for ‘Calandro’ (by 22%) and significantly lower for ‘Bronner’ (by 19%). INF was lower at stage 65 than at the other two stages (Table 2).
Overall, the variability (as indicated by the coefficient of variability, CV) among grapevine varieties was high for SPOR’, LS, and SPOR but was low for LP50 (Table 1).
Relationships among resistance components
As indicated by the correlation matrix (Table 3), there was a significant (P < 0.05) relationship between IFR (and AUIPC as a consequence), LP50, IP, and INF, indicating that as IFR decreased, LP50 increased and IP and INF decreased. LS was significantly (P = 0.032) correlated with LP50, so that LS at 11 dpi increased as the time for the lesions to appear shortened. SPOR’, in contrast, was not correlated with any of the other RCs, with the exception for SPOR.
Cluster analysis
The hierarchical cluster analysis grouped the varieties at different rescaled distances (Fig. 2); at the intermediate distance, four clusters were identified. Based on the OIV degree of resistance (standard OIV 452-1), these clusters represent “high resistance” (CLU1, with an average degree of resistance OIV = 7.0), “medium resistance” (CLU2, OIV = 5.4), “low resistance” (CLU3, OIV = 3.3), and “very low resistance” (CLU4, OIV = 1.5); no varieties showed a “very high” degree of resistance (i.e., with OIV = 9). The average values for each RC of each cluster are shown in Table 4. The among-cluster ANOVA revealed significant differences (P < 0.002) for IFR, LP50, SPOR, and INF, but not for IP (P = 0.07). CLU1 included ‘Bronner’, ‘Johanniter’, and ‘Merlot Kanthus’; CLU1 had the lowest IFR value, the longest LP50, the lowest SPOR, the shortest IP, and the smallest INF. In contrast, CLU4, which included the susceptible control ‘Merlot’ and ‘Rkatsitelii’, had the highest IFR, the shortest LP50, and the highest SPOR. CLU3 contained five varieties (‘Palava’, ‘Reberger’, ‘Felicia’, ‘Calandro’, and ‘Regent’) and had higher IFR, SPOR, and INF values than CLU2, which contained six varieties (‘Merlot Khorus’, ‘Cabernet Volos’, ‘Villaris’, ‘Fleurtai’, ‘Solaris’, and ‘Calardis Blanc’).
Dendrogram resulting from a hierarchical cluster analysis of the five components of partial resistance (RCs) to Plasmopara viticola measured in the grapevine varieties described in Table 5. The RCs are infection frequency (IFR), latency period (LP50), sporulation (SPOR), infectious period (IP), and the infectivity of the sporangia produced on the variety and inoculated on Merlot (INF). Four clusters were identified in the dendrogram (CLU1 to CLU4).
CLU4 contained two varieties without Rpvs, while CLU3 contained varieties without Rpvs or with Rpv3. Rpv3 was also present, alone or pyramided with other Rpvs, in two varieties of CLU2 and two of CLU1. Similarly, Rpv10 was present in two varieties belonging to CLU1 and to CLU2, respectively. The three varieties with Rpv12 were all in CLU2. According to ANOVAs, the RCs IFR, LP50, IP, and INF were not significantly affected by the presence of a particular Rpv in the genome (P > 0.05, Supplementary Table S2). On the contrary, SPOR was significantly higher in the susceptible control ‘Merlot’ than in the varieties with no Rpvs and in the partially resistant varieties carrying Rpv3, Rpv10, or Rpv12; SPOR did not significantly differ among partially resistant varieties carrying Rpv3, Rpv10, or Rpv12 (Fig. 3).
Number of sporangia per downy mildew lesion (SPOR) measured on the grapevine varieties described in Table 5. Varieties were assigned to the following five groups: susceptible control ‘Merlot’ (test), resistant varieties with no Rpv (none), or with Rpv3, Rpv10 or Rpv12. Values are means of data collected in a 3-year period and at three growth stage of grapevines, with 75 replicate leaf discs for each variety in each experiment + SE. Values with different letters are significantly different according to the LSD test (P = 0.05).
Discussion
The purpose of the present research was to characterize the partial resistance to downy mildew (DM) in 15 grapevine varieties, most of which carried one Rpv from V. rupestris or V. amurensis14,47,48,49. Resistance was assessed relative to a susceptible control, ‘Merlot’. The study was conducted by inoculating leaf discs with P. viticola, which is a widely used bioassay in the study of genetic resistance to DM in grapevines7,9,19,50,51,52. Inoculations were performed by using a suspension of P. viticola sporangia derived from sporangia collected in multiple vineyards and then maintained on the susceptible variety ‘Merlot’ for the duration of the study. A population of P. viticola was used instead of single isolates in order to minimize the effect of host–strain genotype interaction on the phenotypic evaluation of the resistance components53,54,55.
The study assessed six main resistance components (RCs) that cover the entire disease monocycle and that do not overlap in terms of processes26,31. RCs were expressed according to their proper dimensions (e.g., frequency for IFR, thermal time for LP50, or numbers of sporangia for SPOR).
IFR was measured as the proportion of inoculated sites that produced DM lesions in the leaf discs assay. IFR is a proxy of infection efficiency (IE), which has been measured as the number of DM lesions generated by each zoospore deposited on the leaf surface56. IE was not used in the current study because its measurement is too complex56 given the number of experiments that were performed. In addition, IE is biased because the number of zoospores produced per sporangium is variable56, multiple germ tubes (from multiple zoospores) can penetrate the same stoma57, and a single lesion may result from multiple penetrations23. Reduction of infection efficiency (measured as IFR in this work) reflects the ability of the plant to reduce the number of pathogen propagules that successfully go through the different steps of the infection process, e.g., the ability of the plant to prevent germ tube penetration of stomata, the formation of substomatal vesicles, the growth of primary and secondary hyphae in the mesophyll, or the growth and functioning of haustoria. IFR can involve both pre- and post-infection mechanisms, such as callose deposition in stomata58,59,60, the presence of inner cuticular rims22, and a HR14,60,61.
The duration of the latent period indicates the time required for the pathogen to produce sporangia on the lesion surface. A longer latent period may result from reduced hyphal growth in the mesophyll of the host plant due to the thickness of the spongy mesophyll and cell walls4,22,62. A longer latent period may also result from a reduced number or functioning of haustoria resulting from HR14, such that the flow of nutrients from host cells to the intercellular mycelium is reduced. AUIPC was also calculated to account for the observation that DM lesions appeared later in partially resistant varieties than in the susceptible control. AUIPC is therefore related to the duration of the incubation period, which is the time required for lesions to appear. The incubation period is not a resistance component sensu Zadoks31 or Parlevliet40, because it does not affect the epidemic development and it is part of the latent period.
The other RCs (i.e., the number of the sporangia produced per DM lesion and per area of DM lesion, the duration of the infectious period, and the infectivity of the sporangia produced on DM lesions) may also be related to a reduced flow of nutrients from the host to the pathogen. For instance, reduced sporangiophore density and reduced numbers of sporangia produced per unit of leaf area were observed in ‘Bianca’ as a consequence of an HR14. The size of DM lesions also reflects the ability of the host to limit the growth of the pathogen in the mesophyll. The synthesis of resveratrol and flavonoids, the increased peroxidase activity, and the formation of lignin in the host tissue all restrict the development of P. viticola and result in smaller lesions63.
INF was not previously considered in analyses of resistance components31, but reduced INF may slow the progress of DM epidemics in partially resistant varieties. The infectivity of P. viticola sporangia produced on DM lesions on leaves of partially resistant varieties has been poorly studied. However, finding similar to those for DM in the current study were described for leaf rust (Puccinia triticinia) on wheat64. Delmotte et al.55 observed that cross-inoculation with the sporangia produced on the partially resistant variety ‘Regent’ on the susceptible cultivar ‘Cabernet Sauvignon’ resulted in higher disease severity, higher sporangia production, and smaller sporangia than inoculation with sporangia produced on the susceptible variety. This seems in contrast with the findings of the current study, in which the sporangia produced on ‘Regent’ had lower infectivity on the susceptible ‘Merlot’ than the sporangia produced on ‘Merlot’. These contrasting results might be explained by differences in methods or in the genotypes of both the host plant and the pathogen.
RCs were expressed to different degrees among the 15 varieties, with significant differences relative to the susceptible ‘Merlot’. High among-variety variability was found for SPOR’, LS, and SPOR (i.e., the combination of LS and SPOR’), and low among-variety variability was found for IP. As expected, lower infection frequency, longer latent period, reduced sporulation, shorter infectious period, and lower infectivity of sporangia were found in the partially resistant varieties than in the susceptible ‘Merlot’. Changes in some of these RCs were significantly correlated, so that varieties with low IFR values also had long LP50 values and low IP and INF values (Table 3). Similar results were found for yellow rust (Puccinia striiformis f. sp. hordei) on barley33, leaf rust (Puccinia triticina) on wheat36, and Phoma black stem of sunflower38, indicating that the mechanisms of resistance act on different stages of the infection process, as previously discussed. Surprisingly, sporulation was not correlated with the other RCs, and this requires further investigation.
Expression of resistance components was generally highest in the varieties carrying Rpv3, Rpv10 or Rpv12, which all grouped in the clusters of high and medium resistance (based on the OIV standards), i.e. CLU1 and CLU2, respectively; the exceptions were ‘Felicia’, ‘Calandro’, and ‘Regent’, which were in the cluster with low resistance (i.e. CLU3), even though they carry Rpv3 (Fig. 2; Table 4). Unfortunately, no association was found between RCs and the Rpvs considered in this work, which would been helpful in breeding programs. On the one hand, the highest level of resistance was found in ‘Bronner’, ‘Merlot Kanthus’ and ‘Johanniter’ (belonging to the same CLU1). ‘Bronner’ (carrying Rpv10) and ‘Johanniter’ (Rpv3) are known to be resistant to DM10,65,66, and both show an HR to P. viticola that leads to cell necrosis and reduced production of sporangia14,67. ‘Merlot Kanthus’ also carries Rpv3. On the other hand, ‘Calandro’, ‘Regent’, and ‘Felicia’ also carry Rpv3, but the resistance of these varieties was low in this work, as already observed in other studies3,10,65. The resistance expressed by ‘Solaris’ (CLU2, medium resistance level) involves both callose synthesis and an HR60. The varieties ‘Merlot Khorus’, ‘Cabernet Volos’, and ‘Fleurtai’ carry the Rpv12 (grouped in CLU2), inherited from V. amurensis49. A typical resistance response of V. amurensis consists in the activation of physical mechanisms involving callose deposition15,58 that leads to the degeneration of portions of mycelium and the alteration of sporangiophore shape7. ‘Rkatsitelii’, a Georgian grapevine variety, showed a very low level of resistance to DM, confirming the results of Bitsadze et al.68.
Overall, our findings indicate that the routine measurement of RCs in breeding programs can be simplified by the measurement of only IFR and SPOR. Table 4 shows that the assessment of IFR made it possible to distinguish CLU2 from CLU1 (medium and high resistance level, respectively), and from CLU4 (very low resistance level), but not CLU3 (low resistance level) from CLU4. At the same time, SPOR made it possible to distinguish CLU4 from CLU3. Therefore, IFR and SPOR, together, were able to distinguish all the four clusters. In addition, since IFR and SPOR are the major drivers of DM epidemic progress in vineyards69, which is caused by the concatenation of infection cycles, the phenotyping of partial resistant varieties by using these two RCs in monocyclic experiments would also account for their effect on epidemic development. This would reduce the workload of phenotyping while still producing correct assessment of resistance.
The current research documented differences in resistance expression among growth stages, with lowest resistance at stage 18, i.e., at leaf development. To our knowledge, no previous studies have reported this growth stage-related variability, and such variability requires further investigation. It is well known from Van der Plank’s25 studies of resistance to potato late blight (Phytophthora infestans) that resistance responses are inducible, which suggests that the expression of resistance in response to attack might be costly70. The metabolic cost of defence may be a form of allocation cost, in which resources devoted to self-protection are not available for other activities such as growth or reproduction71. Because fungicides must be used to protect even partially resistant grapevine varieties against DM51,72, a better understanding of the temporal dynamics of resistance expression may be useful in scheduling these fungicide applications.
Materials and Methods
Plant material
Fifteen partially resistant grapevine varieties and the V. vinifera variety ‘Merlot’, which is highly susceptible to downy mildew and served as a positive control, were used. The varieties, their pedigree, and their Rpvs are listed in Table 5. ‘Calardis Blanc’, ‘Felicia’, ‘Villaris’, ‘Calandro’, ‘Regent’, and ‘Reberger’ were developed by the Julius Kühn Institut (JKI) in Geilweilerhof, Siebeldingen (Germany). ‘Bronner’, ‘Johanniter’, and ‘Solaris’ were developed at the Institute of Viticulture and Enology in Freiburg (Germany). ‘Merlot Khorus’, ‘Merlot Kanthus’, ‘Cabernet Volos’, and ‘Fleurtai’ were developed at the University of Udine and Institute of Applied Genetics (IGA) in Italy.
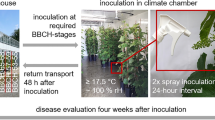
These partially resistant varieties and the positive control ‘Merlot’ were grown in three experimental vineyards in Northern Italy; one vineyard was in Ferrara di Monte Baldo (45°41′5.23′′N 10°51′52.81′′E), and two were in Piacenza (PC, 35°01′32.76′′N 9°39′09.82′′E and 45°02′05.83′′N 9°43′46.41′′E). In 2014, these vineyards were 3, 3, and 2 years old, respectively. Each variety was managed with a single Guyot training system and without fungicide treatment for the duration of the experiment. The experiment was conducted for a 3-year period, from 2014 to 2016.
In each vineyard and year, five plants of each variety were randomly selected at three growth stages: (i) shoot growing, (ii) flowering, and (iii) fruit development. These corresponded to the growth stages 18, 65, and 79, respectively, of Lorenz et al.73. At each growth stage, the fourth leaf from the apex of an actively growing shoot of each plant was sampled74. The detached leaves were then pooled, yielding 15 leaves per variety per stage.
The leaves were placed in a cooler (about 5 °C) and immediately transported to the laboratory where they were washed under tap water, disinfested with sodium hypochlorite (1%) for 1 minute, triple-rinsed using sterile demineralized water, and finally arranged under a sterile laminar flow until their surfaces were completely dry. Following Staudt & Kassemeyer75 and Rumbolz et al.76, 5 leaf discs, 21 mm in diameter, were cut from each leaf using a cork borer, so that 75 leaf discs were obtained for each variety (15 leaves, 5 leaf discs per leaf). The leaf discs were placed abaxial side up in Petri dishes (9 cm diameter) containing two layers of filter paper moistened with 3 ml of sterile demineralized water; the leaf discs were placed on nylon mesh to prevent direct contact with the moistened paper.
Inoculum preparation
For the preparation of the inoculum to be used for artificial inoculations, field-produced sporangia were randomly collected and pooled with the aim of obtaining a diverse natural population of P. viticola sporangia and thereby minimizing the effect of a possible host × strain interaction on the phenotypic evaluation of the resistance components54. To obtain these sporangia, field-grown grape leaves showing typical DM lesions with fresh and abundant sporulation were collected in the spring of 2014 from several vineyards that had not been sprayed with fungicides; these leaves were collected from different V. vinifera varieties and from various locations in Northern Italy. The pooled leaves were brought to the laboratory, where sporangia were collected from lesions (with the help of a needle) and suspended in sterile double-distilled water. Droplets (10 μl) of this bulk suspension of sporangia were placed on the abaxial side of young, fresh leaves that had been detached from potted plants of ‘Merlot’ grown under isolation in a greenhouse at the University of Piacenza. The inoculated leaves were incubated for 7 days in a growth chamber at 20 °C and with high humidity and a 12-h photoperiod. The fresh sporangia produced on the DM lesions were suspended in sterile water, and the suspension was adjusted to 5 × 105 sporangia ml−1. Immediately after preparation, these sporangial suspensions were used for the artificial inoculations described in the next section. The same suspensions were used to maintain the inoculum through repeated inoculations of Merlot leaves, using the same previously described method.
Inoculations
For inoculation of the leaf discs in Petri dishes, four 10-µl droplets of sporangial suspension were applied to each leaf disc using a micropipette. Petri dishes containing the inoculated leaf discs were then sealed with Parafilm (to maintain a saturated atmosphere) and incubated for 24 h in a growth chamber at 20 °C and with a 12-h photoperiod. The inoculum drops were then dried using blotting paper but without touching the leaf surface46. Petri dishes were then sealed again and kept in the growth chamber until the components of partial resistance were measured.
Assessment of resistance
The degree of resistance was visually assessed 11 days post inoculation (dpi) using a modified OIV descriptor 452-146. Leaf discs were rated from the most susceptible to the most resistant as follows: 1, dense sporulation at all four inoculation sites (i.e., very low degree of resistance); 3, dense sporulation at two or three inoculation sites (low resistance); 5, sparse sporulation at two or three inoculation sites (medium resistance); 7, sparse sporulation at one inoculation site (high resistance); and 9, absence of sporulation (very high resistance).
Measurement of the components of partial resistance
Leaf discs were examined daily with a stereomicroscope at 10-fold magnification for the assessment of the RCs as described in the following paragraphs. The 10-fold magnification allowed the screening of a high number of leaf discs with a resolution adequate to observe single sporangiophores of P. viticola50. No new DM signs appeared before 4 dpi or after 11 dpi.
Infection frequency
IFR was assessed at 11 dpi as the proportion of inoculation sites (i.e., sites where the inoculum drops were placed) showing typical DM lesions with sporulation77.
To account for the delay in lesion appearance following inoculation, the AUIPC was calculated by using the daily assessments of IFR between 4 and 11 dpi as follows:
where: \(({y}_{i}+\,{y}_{i+1})\) represents the sum of two consecutive values of IFR, and \(({t}_{i+1}-\,{t}_{i})\) is the time interval between two consecutive assessments. The calculation follows the trapezoidal method for the estimation of the AUDPC (Area Under the Disease Progress Curve), which enables the discretization of the time variable and the calculation of the average disease intensity between each pair of adjacent time points78.
Duration of the latent period
The duration of the latent period (i.e., the time elapsed between inoculation and the start of sporulation on DM lesions) was measured as the thermal time79, i.e., as degree-days (DDs, base 0 °C) accumulated between the inoculation time and the time when 50% of the inoculation sites resulted in DM lesions at the end of the observation period (i.e., at 11 dpi); this is termed the LP5080. For instance, if IFR = 0.25 at 6 dpi (DD = 120, i.e., 6 days at 20 °C) and IFR = 0.75 at 7 dpi (DD = 140) on a leaf disc, then IFR = 0.5 is assumed to be at 6.5 dpi, corresponding to 130 DD; therefore, LP50 = 130.
Lesion size
At 11 dpi, a random sample of 50 leaf discs per variety, which have been inoculated in different growth stages and years, was photographed (13 megapixel f/1.9 resolution), and the area (in mm2) of each lesion (referred as LS) was determined using Assess 2.0 (Image analysis software for plant disease quantification, by Lakhdar Lamari, APS PRESS, Saint Paul, Minnesota). Example of calculation of the lesion size of downy mildew lesions on leaf discs is provided in Supplementary Fig. S1.
Production of sporangia
Production of sporangia was determined as the number of sporangia produced per DM lesion (SPOR) and per mm2 of DM lesion (SPOR’) at 11 dpi. Sporangia were carefully collected from each leaf disc by using a needle; the collected sporangia were suspended in 100 µl of sterile-demineralized water; the suspension was shaken for 10 s, and the sporangia were counted using a hemocytometer. The total number of sporangia was then divided by the number of DM lesions assessed at 11 dpi on each leaf disc (for SPOR), and by the area of each lesion (for SPOR’).
Duration of the infectious period
After 11 dpi, sporulating structures (sporangiophores and sporangia) were gently removed from leaf discs, which have been inoculated at fruit development in the second year, using a sterile cotton swab. Leaf discs were then incubated as previously described and observed every 2 days with a stereomicroscope (10 × magnification). When sporangiophores bearing sporangia re-appeared on lesions, sporulating structures were removed as previously described and the leaf discs were incubated again. This procedure was repeated until the lesions stopped producing new sporangia. The duration of the infectious period (IP, i.e., the period during which a lesion is fertile and continues to produce sporangia) was then expressed as the number of times the lesions produced sporangia81,82.
Infectivity of the sporangia produced on DM lesions
INF was assessed as the ability of the sporangia produced on DM lesions to cause infection on leaves of the susceptible variety ‘Merlot’. At 11 dpi, sporangia produced on the DM lesions obtained in the second year were suspended in water as previously described for inoculum preparation, and the suspension was adjusted to 103 sporangia/ml. These sporangial suspensions were used to inoculate 15 leaf discs excised from ‘Merlot’ leaves that were collected and maintained as previously described. At 11 dpi, INF was assessed as the proportion of inoculation sites (i.e., sites where the inoculum drops have been placed) showing typical DM lesions with sporulation.
Data analysis
The ANOVA was performed to determine whether RCs were significantly affected by grapevine variety, growth stage of vines, year (all considered as fixed factors), and their interactions. Before ANOVAs were conducted, SPOR and SPOR’ data were transformed using the natural logarithm to stabilize variances and were then back-transformed using the inverse exponential function. When F values were significant, means were compared using the Fisher’s protected least significant difference (LSD) post hoc test at P = 0.05.
To study the relationships among RCs, Pearson correlation coefficients were calculated between IFR versus AUIPC, LP50, LS, SPOR’, SPOR, IP, and INF.
To study the relationships between RCs and Rpvs, and among Rpvs, an ANOVA was performed by assigning the varieties into the following groups: susceptible control; varieties with no Rpvs; with Rpv3 (whether Rpv31 and Rpv32); with Rpv10; or with Rpv12.
A hierarchical cluster analysis was conducted for grouping grapevine varieties based on the within-group similarities and between-group differences in the average values of five RCs: IFR, LP50, SPOR, IP, and INF. AUIPC was not included in this analysis because it was closely correlated to IFR (see Table 3); LS and SPOR’ were also not included because SPOR incorporates the information concerning the lesion size and the sporulation per surface unit of lesions. The Ward’s method was used for clustering, in which the distance between two clusters is indicated by the increase in the sum of squares caused by merging of the clusters, with the Euclidean square distance for measuring similarities. Because the RC values were measured on different scales, some being much larger than others, the data were standardized by using the z-scores as follows: \(({{\rm{x}}}_{{\rm{i}}}-\bar{{\rm{x}}})/{\rm{sd}}\), where \({{\rm{x}}}_{{\rm{i}}}\) is any value of a variable, \(\bar{{\rm{x}}}\) is the average for the variable, and sd is the standard deviation. An ANOVA was then performed to determine whether the groups of varieties formed by the cluster analysis were significantly different for each of the five RCs.
All analyses were performed using IBM SPSS software version 24 (SPSS, Chicago, IL).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gessler, C., Pertot, I. & Perazzolli, M. Plasmopara viticola: A review of knowledge on downy mildew of grapevine and effective disease management. Phytopathol. Mediterr. 50, 3–44 (2011).
Millardet, A. Notes sur les vignes américaines et opuscules divers sur le même sujet (1881).
Boso, S. & Kassemeyer, H. H. Different susceptibility of European grapevine cultivars for downy mildew. Vitis 47, 39–49 (2008).
Alonso-Villaverde, V., Voinesco, F., Viret, O., Spring, J. L. & Gindro, K. The effectiveness of stilbenes in resistant Vitaceae: Ultrastructural and biochemical events during Plasmopara viticola infection process. Plant Physiol. Biochem. 49, 265–274 (2011).
Alleweldt, G. & Possingham, J. V. Progress in grapevine breeding. Theor. Appl. Genet. 75, 669–673 (1988).
Moreira, F. M. et al. Genetic linkage maps of two interspecific grape crosses (Vitis spp.) used to localize quantitative trait loci for downy mildew resistance. Tree Genet. Genomes 7, 153–167 (2011).
Toffolatti, S. L. et al. Unique resistance traits against downy mildew from the center of origin of grapevine (Vitis vinifera). Sci. Rep. 8, 1–11 (2018).
Di Gaspero, G., Cipriani, G., Adam-Blondon, A. F. & Testolin, R. Linkage maps of grapevine displaying the chromosomal locations of 420 microsatellite markers and 82 markers for R-gene candidates. Theor. Appl. Genet. 114, 1249–1263 (2007).
Calonnec, A. et al. The reliability of leaf bioassays for predicting disease resistance on fruit: A case study on grapevine resistance to downy and powdery mildew. Plant Pathol. 62, 533–544 (2013).
Vezzulli, S., Vecchione, A., Stefanini, M. & Zulini, L. Downy mildew resistance evaluation in 28 grapevine hybrids promising for breeding programs in Trentino region (Italy). Eur. J. Plant Pathol. 150, 485–495 (2018).
Alleweldt, G. The breeding of fungus- and phylloxera-resistant grapevine varieties. In 3rd Int. Symp. Grape Breeding 242––250 (Dept. of Vitic. and Enology, 1980).
Bouquet, A. Vitis x Muscadinia hybridization: a new way in grape breeding for disease resistance in France. In 3rd Int. Symp. Grape Breeding 42–61 (Dept. of Vitic. and Enology, 1980).
Kozma, P. Goals and methods in grape resistance breeding in Hungary. Int. J. Horticoutural Sci. 8, 41–46 (2002).
Bellin, D. et al. Resistance to Plasmopara viticola in grapevine ‘Bianca’ is controlled by a major dominant gene causing localised necrosis at the infection site. Theor. Appl. Genet. 120, 163–176 (2009).
Yu, Y., Zhang, Y., Yin, L. & Lu, J. The Mode of Host Resistance to Plasmopara viticola Infection of Grapevines. Phytopathology 102, 1094–1101 (2012).
Atak, A. Determination of downy mildew and powdery mildew resistance of some grape cultivars. South African J. Enol. Vitic. 38, 11–17 (2017).
Gómez-Zeledón, J., Kaiser, M. & Spring, O. Exploring host-pathogen combinations for compatible and incompatible reactions in grapevine downy mildew. Eur. J. Plant Pathol. 149, 1–10 (2017).
He, R. et al. Overexpression of a thaumatin-like protein gene from Vitis amurensis improves downy mildew resistance in Vitis vinifera grapevine. Protoplasma 254, 1579–1589 (2017).
Sánchez-Mora, F. D. et al. Behavior of grape breeding lines with distinct resistance alleles to downy mildew (Plasmopara viticola). Crop Breed. Appl. Biotechnol. 17, 141–149 (2017).
Yin, L. et al. Genome sequence of Plasmopara viticola and insight into the pathogenic mechanism. Sci. Rep. 7, 1–12 (2017).
Zhang, Y. et al. Genome-wide assessment of population structure, linkage disequilibrium and resistant QTLs in Chinese wild grapevine. Sci. Hortic. (Amsterdam). 215, 59–64 (2017).
Jürges, G., Kassemeyer, H. H., Dürrenberger, M., Düggelin, M. & Nick, P. The mode of interaction between Vitis and Plasmopara viticola Berk. & Curt. Ex de Bary depends on the host species. Plant Biol. 11, 886–898 (2009).
Polesani, M. et al. General and species-specific transcriptional responses to downy mildew infection in a susceptible (Vitis vinifera) and a resistant (V. Riparia) grapevine species. BMC Genomics 11, 117 (2010).
Brilli, M. et al. A multi-omics study of the grapevine-downy mildew (Plasmopara viticola) pathosystem unveils a complex protein coding- and noncoding-based arms race during infection. Sci. Rep. 8, 757 (2018).
Van der Plank, J. E. Plant Diseases: Epidemics and Control. (Academic Press Inc. (New York), 1963).
Willocquet, L., Savary, S. & Yuen, J. Multiscale Phenotyping and Decision Strategies in Breeding for Resistance. Trends Plant Sci. 22, 420–432 (2017).
Ricker, M. D., Beute, M. K. & Campbell, C. L. Components of Resistance in Peanut to Cercospora arachidicola. Plant Dis. 69, 1059–1064 (1985).
Waliyar, F., McDonald, D., Subba Rao, P. V. & Reddy, P. M. Components of Resistance to an Indian Source of Cercospora Arachidicola in Selected Peanut Lines. Peanut Sci. 20, 93–96 (1993).
Rossi, V. et al. Components of rate-reducing resistance to Cercospora leaf spot in sugar beet: conidiation length, spore yield. J. Plant Pathol. 82, 125–131 (2000).
Gordon, S. G., Lipps, P. E. & Pratt, R. C. Heritability and components of resistance to Cercospora zeae-maydis derived from maize inbred VO613Y. Phytopathology 96, 593–598 (2006).
Zadoks, J. C. Modern concepts of disease resistance in cereals. In The Way Ahead in Plant Breeding. (The Plant Breeding Institute, 1972).
Rossi, V. et al. Components of rate-reducing resistance to Cercospora leaf spot in sugar beet: incubation length, infection efficiency, lesion size. J. Plant Pathol. 81, 25–35 (1999).
Sandoval-Islas, J. S. et al. Quantitative resistance and its components in 16 barley cultivars to yellow rust, Puccinia striiformis f. sp. hordei. Euphytica 153, 295–308 (2007).
Villareal, R. L., Nelson, R. R., MacKenzie, D. R. & Coffman, W. R. Some Components of Slow-Blasting Resistance in Rice. Phytopathology 71, 608–611 (1981).
Willocquet, L., Lore, J. S., Srinivasachary, S. & Savary, S. Quantification of the Components of Resistance to Rice Sheath Blight Using a Detached Tiller Test Under Controlled Conditions. Plant Dis. 95, 1507–1515 (2011).
Herrera-Foessel, S. A. et al. Evaluation of slow rusting resistance components to leaf rust in CIMMYT durum wheats. Euphytica 155, 361–369 (2007).
Burlakoti, R. R., Mergoum, M., Kianian, S. F. & Adhikari, T. B. Combining different resistance components enhances resistance to Fusarium head blight in spring wheat. Euphytica 172, 197–205 (2010).
Schwanck, A. A. et al. Predicting quantitative host plant resistance against phoma black stem in sunflower. Plant Pathol. 65, 1366–1379 (2016).
Van der Plank, J. E. Disease resistance in plants. (Academic Press, 1968).
Parlevliet, J. E. Components of Resistance that Reduce the Rate of Epidemic Development. Annu. Rev. Phytopathol. 17, 203–222 (1979).
Savary, S. & Willocquet, L. Simulation Modeling in Botanical Epidemiology and Crop Loss Analysis. The Plant Health Instructor 173 (2014).
Bove, F. A modelling framework for grapevine downy mildew epidemics incorporating foliage-cluster relationships and host plant resistance. (Università Cattolica del Sacro Cuore, Piacenza, Italy, 2018).
Savary, S., De Jong, P. D., Rabbinge, R. & Zadoks, J. C. Dynamic simulation of groundnut rust: a preliminary model. Agric. Syst. 32, 113–141 (1990).
Van Oijen, M. Selection and use of a mathematical model to evaluate components of resistance to Phytophthora infestans in potato. Netherlands J. Plant Pathol. 98, 192–202 (1992).
Luo, Y. & Zeng, S. M. Simulation studies on epidemics of wheat stripe rust (Puccinia striiformis) on slow-rusting cultivars and analysis of effects of resistance components. Plant Pathol. 44, 340–349 (1995).
OIV. 2nd Edition of the OIV descriptor list for grape varieties and Vitis species (Organisation Internationale de la Vigne et du Vin, 2009).
Welter, L. J. et al. Genetic mapping and localization of quantitative trait loci affecting fungal disease resistance and leaf morphology in grapevine (Vitis vinifera L). Mol. Breed. 20, 359–374 (2007).
Schwander, F., Eibach, R., Zyprian, E. & Reinhard, T. Localisation and fine-mapping of the downy mildew resistance locus Rpv10 in grapevine. In Nachwuchswissenschaftlerforum 2011 (Julius Kühn-Institut, 2011).
Venuti, S. et al. Historical Introgression of the Downy Mildew Resistance Gene Rpv12 from the Asian Species Vitis amurensis into Grapevine Varieties. PLoS One 8, e61228 (2013).
Schwander, F. et al. Rpv10: a new locus from the Asian Vitis gene pool for pyramiding downy mildew resistance loci in grapevine. Theor. Appl. Genet. 124, 163–176 (2012).
Delmas, C. E. L. et al. Adaptation of a plant pathogen to partial host resistance: Selection for greater aggressiveness in grapevine downy mildew. Evol. Appl. 9, 709–725 (2016).
Ochssner, I., Hausmann, L. & Töpfer, R. Rpv14, a new genetic source for Plasmopara viticola resistance conferred by Vitis cinerea. Vitis - J. Grapevine Res. 55, 79–81 (2016).
Peressotti, E. et al. Breakdown of resistance to grapevine downy mildew upon limited deployment of a resistant variety. BMC Plant Biol. 10, 147 (2010).
Gómez Zeledón, J., Zipper, R. & Spring, O. Assessment of phenotypic diversity of Plasmopara viticola on Vitis genotypes with different resistance. Crop Prot. 54, 221–228 (2013).
Delmotte, F. et al. Rapid and multiregional adaptation to host partial resistance in a plant pathogenic oomycete: Evidence from European populations of Plasmopara viticola, the causal agent of grapevine downy mildew. Infect. Genet. Evol. 27, 500–508 (2014).
Lalancette, N. Estimating Infection Efficiency of Plasmopara viticola on Grape. Plant Dis. 71, 981 (1987).
Amici, A., Baldacci, E. & Minervini, G. Sulla struttura e sulla modalità di penetrazione dell’austorio di Plasmopara viticola in Vitis vinifera (Rilievi di microscopia elettronica). Riv. di Patol. Veg. 4, 165–184 (1968).
Kortekamp, A., Wind, R. & Zyprian, E. The role of callose deposits during infection of two downy mildew-tolerant and two -susceptible Vitis cuitivars. Vitis 36, 103–104 (1997).
Kortekamp, A., Wind, R. & Zyprian, E. Investigation on the interaction of Plasmopara viticola with susceptible and resistant grapevine cultivars. J. Plant Dis. Prot. 105, 475–488 (1998).
Gindro, K., Pezet, R. & Viret, O. Histological study of the responses of two Vitis vinifera cultivars (resistant and susceptible) to Plasmopara viticola infections. Plant Physiol. Biochem. 41, 846–853 (2003).
Dercks, W. & Creasy, L. L. The significance of stilbene phytoalexins in the Plasmopara viticola-grapevine interaction. Physiol. Mol. Plant Pathol. 34, 189–202 (1989).
Muganu, M. & Paolocci, M. Adaptation of Local Grapevine Germplasm: Exploitation of Natural Defence Mechanisms to Biotic Stresses. Mediterr. Genet. Code - Grapevine Olive, https://doi.org/10.5772/51976 (2013).
Dai, G. H., Andary, C., Mondolot-Cosson, L. & Boubals, D. Histochemical responses of leaves of in vitro plantlets of Vitis spp. to infection with Plasmopara viticola. Phytopathology 85, 149–154 (1995).
Lehman, J. S. & Shaner, G. Heritability of latent period estimated from wild-type and selected populations of Puccinia triticina. Phytopathology 97, 1022–1029 (2007).
Basler, P. & Pfenninger, H. Disease-resistant cultivars as a solution for organic viticulture. Acta Hortic. 603, 681–685 (2003).
Pezet, R., Gindro, K., Viret, O. & Spring, J. L. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 65, 297–303 (2004).
Casagrande, K., Falginella, L., Castellarin, S. D., Testolin, R. & Di Gaspero, G. Defence responses in Rpv3-dependent resistance to grapevine downy mildew. Planta 234, 1097–1109 (2011).
Bitsadze, N. et al. Screening of Georgian grapevine germplasm for susceptibility to downy mildew (Plasmopara viticola). Vitis - J. Grapevine Res. 54, 193–196 (2015).
Bove, F., Bavaresco, L., Caffi, T. & Rossi, V. Assessment of Resistance Components for Improved Phenotyping of Grapevine Varieties Resistant to Downy Mildew. Front. Plant Sci. 10, 1–10 (2019).
Vos, I. A., Pieterse, C. M. J. & Van Wees, S. C. M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 62, 43–55 (2013).
Brown, J. K. M. & Rant, J. C. Fitness costs and trade-offs of disease resistance and their consequences for breeding arable crops. Plant Pathol. 62, 83–95 (2013).
Töpfer, R. et al. Fruit, Vegetable and Cereal Science and Biotechnology New Horizons for Grapevine Breeding. New Horizons grapevine Breed. 5, 79–100 (2011).
Lorenz, D. H. et al. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1, 100–103 (1995).
Reuveni, M. Relationships between Leaf Age, Peroxidase and β-1, 3-GIucanase Activity, and Resistance to Downy Mildew in Grapevines. J. Phytopathol. 146, 525–530 (1998).
Staudt, G. & Kassemeyer, H. Evaluation of downy mildew resistance in various accessions of wild Vitis species. Vitis 34, 225–228 (1995).
Rumbolz, J. et al. Sporulation of Plasmopara viticola: differentiation and light regulation. Plant Biol. 4, 413–422 (2002).
Caffi, T., Legler, S. E., González-Domínguez, E. & Rossi, V. Effect of temperature and wetness duration on infection by Plasmopara viticola and on post-inoculation efficacy of copper. Eur. J. Plant Pathol. 144, 737–750 (2016).
Madden, L. V., Hughes, G. & van den Bosch, F. The Study of Plant Disease Epidemics, https://doi.org/10.1094/9780890545058.fm (American Phytopathological Society (APS Press), 2007).
Lovell, D. J., Powers, S. J., Welham, S. J. & Parker, S. R. A perspective on the measurement of time in plant disease epidemiology. Plant Pathol. 53, 705–712 (2004).
Parlevliet, J. E. Partial resistance of barley to leafrust, Puccinia hordei. I. Effect of cultivar and development stage on latent period. Euphytica 24, 21–27 (1975).
Kennelly, M. M., Gadoury, D. M., Wilcox, W. F., Magarey, P. A. & Seem, R. C. Primary infection, lesion productivity, and survival of sporangia in the grapevine downy mildew pathogen Plasmopara viticola. Phytopathology 97, 512–522 (2007).
Caffi, T., Gilardi, G., Monchiero, M. & Rossi, V. Production and release of asexual sporangia in Plasmopara viticola. Phytopathology 103, 64–73 (2013).
Acknowledgements
This research was partially founded by the European collaborative project Innovine, under grant agreement 311775. This study was supported by the Ph.D. in Agro-Food System (Agrisystem) of the Università Cattolica del Sacro Cuore (Italy). We thank Serge Savary and Laetitia Willocquet for their critical review of the manuscript and for their valuable advice.
Author information
Authors and Affiliations
Contributions
Design of the research: V.R.; performance of the research: F.B.; Data collection: F.B.; Data analysis and interpretation: V.R. and F.B.; writing: V.R. and F.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bove, F., Rossi, V. Components of partial resistance to Plasmopara viticola enable complete phenotypic characterization of grapevine varieties. Sci Rep 10, 585 (2020). https://doi.org/10.1038/s41598-020-57482-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57482-0
This article is cited by
-
Plasmopara viticola infection process and accumulation of important stilbenes in some grapevine varieties grown in Turkey and in individuals for brined leaves
Journal of Plant Diseases and Protection (2022)
-
Molecular characterization of the Rpv3 locus towards the development of KASP markers for downy mildew resistance in grapevine (Vitis spp.)
Euphytica (2022)
-
Grapevine Rpv3-, Rpv10- and Rpv12-mediated defense responses against Plasmopara viticola and the impact of their deployment on fungicide use in viticulture
BMC Plant Biology (2021)
-
Modelling the effect of partial resistance on epidemics of downy mildew of grapevine
European Journal of Plant Pathology (2021)
-
Two-omics data revealed commonalities and differences between Rpv12- and Rpv3-mediated resistance in grapevine
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.