Abstract
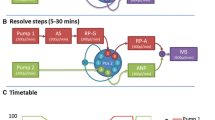
To close the “analytical gap” in the liquid chromatographic (LC) analysis of highly polar substances, two techniques which have been suggested earlier were tested in terms of retention factors and detection limits: hydrophilic interaction liquid chromatography (HILIC) and mixed-mode chromatography (MMC). A substance mix of 55 analytes ranging from logD − 8.2 to 3.4 and 17 different LC columns, also comprising additional reversed-phase columns were used. Contrary to most reversed-phase columns, column bleed has been identified as an important factor, which may cause serious restrictions during high-resolution mass spectrometric detection (HRMS). We found that highly abundant background masses continuously eluting from the columns heavily influence ion transmission to the detector. As a result, the linear dynamic range as well as the sensitivity decreases and thus limits the HRMS applicability of some columns. We therefore recommend a thorough investigation of ion transmission during HRMS method development. This will help to maintain the high potential of HRMS in terms of qualitative and quantitative screening analysis.





Similar content being viewed by others
References
Berger U, Ost N, Sättler D, Schliebner I, Kühne R, Schüürman G, et al. Assessment of persistence, mobility and toxicity (PMT) of 167 REACH registered substances. Umweltbundesamt (UBA) Texte. 2018;9:2018.
Reemtsma T, Berger U, Arp HPH, Gallard H, Knepper TP, Neumann M, et al. Mind the gap: persistent and mobile organic compounds—water contaminants that slip through. Environ Sci Technol. 2016;50(19):10308–15. https://doi.org/10.1021/acs.est.6b03338.
Kalberlah F, Oltmanns J, Schwarz M, Baumeister J, Striffler A. Guidance for the precautionary protection of raw water destined for drinking water extraction from contaminants regulated under REACH. UFOPLAN Project FKZ 371265416. 2014.
Bieber S, Greco G, Grosse S, Letzel T. RPLC-HILIC and SFC with mass spectrometry: polarity-extended organic molecule screening in environmental (water) samples. Anal Chem. 2017;89(15):7907–14. https://doi.org/10.1021/acs.analchem.7b00859.
Trautwein C, Berset J-D, Wolschke H, Kümmerer K. Occurrence of the antidiabetic drug metformin and its ultimate transformation product guanylurea in several compartments of the aquatic cycle. Environ Int. 2014;70:203–12. https://doi.org/10.1016/j.envint.2014.05.008.
ChemAxon. https://chemicalize.com/. Accessed September 2019.
Scheurer M, Nödler K, Freeling F, Janda J, Happel O, Riegel M, et al. Small, mobile, persistent: trifluoroacetate in the water cycle – overlooked sources, pathways, and consequences for drinking water supply. Water Res. 2017;126:460–71. https://doi.org/10.1016/j.watres.2017.09.045.
EuropeanCommission (2018) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on the quality of water intended for human consumption (recast). 2017/0332(COD). Brussels.
Schulze S, Zahn D, Montes R, Rodil R, Quintana JB, Knepper TP, et al. Occurrence of emerging persistent and mobile organic contaminants in European water samples. Water Res. 2019;153:80–90. https://doi.org/10.1016/j.watres.2019.01.008.
Montes R, Aguirre J, Vidal X, Rodil R, Cela R, Quintana JB. Screening for polar chemicals in water by trifunctional mixed-mode liquid chromatography–high resolution mass spectrometry. Environ Sci Technol. 2017;51(11):6250–9. https://doi.org/10.1021/acs.est.6b05135.
Gago-Ferrero P, Schymanski EL, Bletsou AA, Aalizadeh R, Hollender J, Thomaidis NS. Extended suspect and non-target strategies to characterize emerging polar organic contaminants in raw wastewater with LC-HRMS/MS. Environ Sci Technol. 2015;49(20):12333–41. https://doi.org/10.1021/acs.est.5b03454.
Zhang K, Liu X. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J Pharm Biomed Anal. 2016;128:73–88. https://doi.org/10.1016/j.jpba.2016.05.007.
McCalley DV. Understanding and manipulating the separation in hydrophilic interaction liquid chromatography. J Chromatogr A. 2017;1523:49–71. https://doi.org/10.1016/j.chroma.2017.06.026.
Alpert AJ. Effect of salts on retention in hydrophilic interaction chromatography. J Chromatogr A. 2018;1538:45–53. https://doi.org/10.1016/j.chroma.2018.01.038.
Teutenberg T, Tuerk J, Holzhauser M, Kiffmeyer TK (2006) Evaluation of column bleed by using an ultraviolet and a charged aerosol detector coupled to a high-temperature liquid chromatographic system. J Chromatogr A 1119 (1):197–201. doi:https://doi.org/10.1016/j.chroma.2005.12.011.
Wells GJ. Charge control for ionic charge accumulation devices. US Patent No. 2009;7:629,575.
Time-of-flight mass spectrometry - technical overview (2011). Agilent Technologie, Inc., USA.
Scherrer RA, Howard SM. Use of distribution coefficients in quantitative structure-activity relations. J Med Chem. 1977;20(1):53–8. https://doi.org/10.1021/jm00211a010.
Arp HPH, Brown TN, Berger U, Hale SE. Ranking REACH registered neutral, ionizable and ionic organic chemicals based on their aquatic persistency and mobility. Environ Sci Process Impacts. 2017;19(7):939–55. https://doi.org/10.1039/C7EM00158D.
Nödler K, Happel O, Scheurer M, Storck FR, Brauch H-J (2018) Selektion von für die Wasserversorgung relevanten prioritären Stoffen und Erarbeitung einer Stoffliste. DVGW Deutscher Verein des Gas- und Wasserfaches e. V., Bonn.
Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem. 2007;389(4):1017–31. https://doi.org/10.1007/s00216-007-1486-6.
Ipsen A, Ebbels TMD. Orders of magnitude extension of the effective dynamic range of TDC-based TOFMS data through maximum likelihood estimation. J Am Soc Mass Spectrom. 2014;25(10):1824–7. https://doi.org/10.1007/s13361-014-0961-5.
Lagerwerf FM, van Dongen WD, Steenvoorden RJJM, Honing M, Jonkman JHG. Exploring the boundaries of bioanalytical quantitative LC–MS–MS. Trends Anal Chem. 2000;19(7):418–27. https://doi.org/10.1016/S0165-9936(00)00009-1.
Zimmer D. Introduction to quantitative liquid chromatography-tandem mass spectrometry (LC-MS-MS). Chromatographia. 2003;57(1):S325–32. https://doi.org/10.1007/BF02492124.
Funke J, Valkov V, Balsaa P, Schmidt TC Amidosulfonsäure - Quantifizierung eines kleinen, hochpolaren Moleküls mit Reversed-Phase-LC-MS-MS. Poster Jahrestagung der Wasserchemischen Gesellschaft 2019, Erfurt 27 - 29 Mai 2019 ISBN 978-3-947197-11-8.
Boulard L, Dierkes G, Ternes T. Utilization of large volume zwitterionic hydrophilic interaction liquid chromatography for the analysis of polar pharmaceuticals in aqueous environmental samples: benefits and limitations. J Chromatogr A. 2018;1535:27–43. https://doi.org/10.1016/j.chroma.2017.12.023.
Mihailova A, Lundanes E, Greibrokk T. Determination and removal of impurities in 2-D LC-MS of peptides. J Sep Sci. 2006;29(4):576–81. https://doi.org/10.1002/jssc.200500496.
Meier F, Geyer PE, Virreira Winter S, Cox J, Mann M. BoxCar acquisition method enables single-shot proteomics at a depth of 10,000 proteins in 100 minutes. Nat Methods. 2018;15(6):440–8. https://doi.org/10.1038/s41592-018-0003-5.
Keller BO, Sui J, Young AB, Whittal RM. Interferences and contaminants encountered in modern mass spectrometry. Anal Chim Acta. 2008;627(1):71–81. https://doi.org/10.1016/j.aca.2008.04.043.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Persistent and Mobile Organic Compounds – An Environmental Challenge with guest editors Torsten C. Schmidt, Thomas P. Knepper, and Thorsten Reemtsma.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 361 kb)
Rights and permissions
About this article
Cite this article
Schulze, B., Bader, T., Seitz, W. et al. Column bleed in the analysis of highly polar substances: an overlooked aspect in HRMS. Anal Bioanal Chem 412, 4837–4847 (2020). https://doi.org/10.1007/s00216-020-02387-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02387-0