Multiplexed Remote SPR Detection of Biological Interactions through Optical Fiber Bundles
Abstract
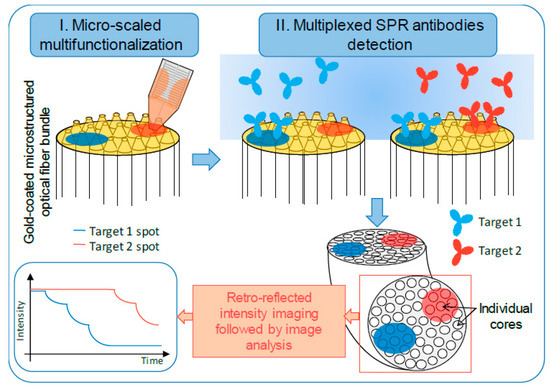
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Optical Fiber Bundle Preparation
2.3. Optical Setup and Characterization
2.4. Surface Biofunctionalization
2.5. Multiplexed Biodetection Assays
3. Results and Discussion
3.1. Optical Setup and Characterization
3.2. Surface Biofunctionalization
3.3. Multiplexed Biodetection Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Laemmel, E.; Genet, M.; Goualher, G.L.; Perchant, A.; Gargasson, J.-F.L.; Vicaut, E. Fibered confocal fluorescence microscopy (Cell-viZio™) facilitates extended imaging in the field of microcirculation. J. Vasc. Res. 2004, 41, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Marazuela, M.; Moreno-Bondi, M. Fiber-optic biosensors—An overview. Anal. Bioanal. Chem. 2002, 372, 664–682. [Google Scholar] [CrossRef] [PubMed]
- López-Higuera, J.M. Handbook of Optical Fibre Sensing Technology; Wiley: Chichester, UK; New York, NY, USA, 2002; ISBN 978-0-471-82053-6. [Google Scholar]
- Monk, D.J.; Walt, D.R. Optical fiber-based biosensors. Anal. Bioanal. Chem. 2004, 379, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Barnard, S.M.; Walt, D.R. A fibre-optic chemical sensor with discrete sensing sites. Nature 1991, 353, 338. [Google Scholar] [CrossRef]
- Mignani, A.G.; Baldini, F. Biomedical sensors using optical fibres. Rep. Prog. Phys. 1996, 59, 1–28. [Google Scholar] [CrossRef]
- Poeggel, S.; Tosi, D.; Duraibabu, D.; Leen, G.; McGrath, D.; Lewis, E. Optical fibre pressure sensors in medical applications. Sensors 2015, 15, 17115–17148. [Google Scholar] [CrossRef]
- Peterson, J.I.; Goldstein, S.R.; Fitzgerald, R.V.; Buckhold, D.K. Fiber optic pH probe for physiological use. Anal. Chem. 1980, 52, 864–869. [Google Scholar] [CrossRef]
- Riva, C.; Ross, B.; Benedek, G.B. Laser Doppler measurements of blood flow in capillary tubes and retinal arteries. Investig. Ophthalmol. 1972, 11, 936–944. [Google Scholar]
- Baldini, F.; Bechi, P.; Bracci, S.; Cosi, F.; Pucciani, F. In vivo optical-fibre pH sensor for gastro-oesophageal measurements. Sens. Actuators B Chem. 1995, 29, 164–168. [Google Scholar] [CrossRef]
- Ahn, S.-M.; Simpson, R.J. Body fluid proteomics: Prospects for biomarker discovery. Proteom. Clin. Appl. 2007, 1, 1004–1015. [Google Scholar] [CrossRef]
- Haslene-Hox, H.; Madani, A.; Berg, K.C.G.; Woie, K.; Salvesen, H.B.; Wiig, H.; Tenstad, O. Quantification of the concentration gradient of biomarkers between ovarian carcinoma interstitial fluid and blood. BBA Clin. 2014, 2, 18–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Andrian, U.H.; Mempel, T.R. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003, 3, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.T.; Jung, S.; Cheng, G.; Weninger, W.; Luo, Y.; Dorf, M.; Littman, D.R.; Rollins, B.J.; Zweerink, H.; Rot, A.; et al. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001, 194, 1361–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Tong, R.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.D.; Kant, R. [INVITED] Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pandey, A.K.; Kaur, B. A review of advancements (2007–2017) in plasmonics-based optical fiber sensors. Opt. Fiber Technol. 2018, 43, 20–34. [Google Scholar] [CrossRef]
- Klantsataya, E.; Jia, P.; Ebendorff-Heidepriem, H.; Monro, T.M.; François, A. Plasmonic fiber optic refractometric sensors: From conventional architectures to recent design trends. Sensors 2016, 17, 12. [Google Scholar] [CrossRef] [Green Version]
- Correia, R.; James, S.; Lee, S.-W.; Morgan, S.P.; Korposh, S. Biomedical application of optical fibre sensors. J. Opt. 2018, 20, 073003. [Google Scholar] [CrossRef]
- Yuan, C.; Lou, Z.; Wang, W.; Yang, L.; Li, Y. Synthesis of Fe3C@C from Pyrolysis of Fe3O4-Lignin clusters and its application for quick and sensitive detection of PrPSc through a sandwich SPR detection assay. Int. J. Mol. Sci. 2019, 20, 741. [Google Scholar] [CrossRef] [Green Version]
- Lou, Z.; Han, H.; Mao, D.; Jiang, Y.; Song, J. Qualitative and quantitative detection of PrPSc based on the controlled release property of magnetic microspheres using surface plasmon resonance (SPR). Nanomaterials 2018, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Luo, Z.; Chen, J.; Huang, Z.; Wang, X.; An, H.; Duan, Y. Ω-Shaped fiber-optic probe-based localized surface plasmon resonance biosensor for real-time detection of Salmonella typhimurium. Anal. Chem. 2018, 90, 13640–13646. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Bea, S.O.; Kim, D.M.; Yoon, W.J.; Park, J.-W.; An, S.S.A.; Ju, H. A regenerative label-free fiber optic sensor using surface plasmon resonance for clinical diagnosis of fibrinogen. Int. J. Nanomed. 2015, 10, 155–163. [Google Scholar]
- Pollet, J.; Delport, F.; Janssen, K.P.F.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic SPR biosensing of DNA hybridization and DNA-protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Sciacca, B.; Monro, T.M. Dip biosensor based on localized surface plasmon resonance at the tip of an optical fiber. Langmuir 2014, 30, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gupta, B.D. SPR based three channels fiber optic sensor for aqueous environment. In Proceedings of the Photonic Instrumentation Engineering, International Society for Optics and Photonics, San Francisco, CA, USA, 1–6 February 2014; Volume 8992, p. 899209. [Google Scholar]
- Bronk, K.S.; Michael, K.L.; Pantano, P.; Walt, D.R. Combined imaging and chemical sensing using a single optical imaging fiber. Anal. Chem. 1995, 67, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.K.; Cavanagh, P.E.; Li, X.; Walt, D.R. Ultrasensitive single-molecule enzyme detection and analysis using a polymer microarray. Anal. Chem. 2018, 90, 3091–3098. [Google Scholar] [CrossRef]
- Walt, D.R. Fibre optic microarrays. Chem. Soc. Rev. 2009, 39, 38–50. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Walt, D.R. Very high density sensing arrays. Chem. Rev. 2008, 108, 614–637. [Google Scholar] [CrossRef]
- Deiss, F.; Sojic, N.; White, D.J.; Stoddart, P.R. Nanostructured optical fibre arrays for high-density biochemical sensing and remote imaging. Anal Bioanal. Chem. 2010, 396, 53–71. [Google Scholar] [CrossRef]
- Vindas, K.; Leroy, L.; Garrigue, P.; Voci, S.; Livache, T.; Arbault, S.; Sojic, N.; Buhot, A.; Engel, E. Highly parallel remote SPR detection of DNA hybridization by micropillar optical arrays. Anal. Bioanal. Chem. 2019, 411, 2249–2259. [Google Scholar] [CrossRef]
- Smythe, E.J.; Dickey, M.D.; Whitesides, G.M.; Capasso, F. A technique to transfer metallic nanoscale patterns to small and non-planar surfaces. ACS Nano 2009, 3, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, J.C.; Alvis, R.M.; Brown, S.B.; Langry, K.C.; Wilson, T.S.; McBride, M.T.; Myrick, M.L.; Cox, W.R.; Grove, M.E.; Colston, B.W. Fabricating optical fiber imaging sensors using inkjet printing technology: A pH sensor proof-of-concept. Biosens. Bioelectron. 2006, 21, 1359–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Descamps, E.; Duroure, N.; Deiss, F.; Leichlé, T.; Adam, C.; Mailley, P.; Aït-Ikhlef, A.; Livache, T.; Nicu, L.; Sojic, N. Functionalization of optical nanotip arrays with an electrochemical microcantilever for multiplexed DNA detection. Lab Chip 2013, 13, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Adam, C.; Sojic, N.; Schmittel, M. Photochemical functionalisation of optical nanotips with a rhodamine chemosensor for remote through-fiber detection of Hg2+. RSC Adv. 2013, 3, 24140–24145. [Google Scholar] [CrossRef]
- Shu, Q.; Adam, C.; Sojic, N.; Schmittel, M. Electrochemiluminescent polymer films with a suitable redox “turn-off” absorbance window for remote selective sensing of Hg2+. Analyst 2013, 138, 4500–4504. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Martinez, R.V.; Kats, M.A.; Kang, S.H.; Kim, P.; Aizenberg, J.; Capasso, F.; Whitesides, G.M. Patterning the tips of optical fibers with metallic nanostructures using nanoskiving. Nano Lett. 2011, 11, 632–636. [Google Scholar] [CrossRef]
- Berthet-Duroure, N.; Leïchlé, T.; Pourciel, J.-B.; Martin, C.; Bausells, J.; Lora-Tamayo, E.; Perez-Murano, F.; François, J.M.; Trévisiol, E.; Nicu, L. Interaction of biomolecules sequentially deposited at the same location using a microcantilever-based spotter. Biomed. Microdevices 2008, 10, 479–487. [Google Scholar] [CrossRef]
- Leichle, T.; Lishchynska, M.; Mathieu, F.; Pourciel, J.; Saya, D.; Nicu, L. A microcantilever-based picoliter droplet dispenser with integrated force sensors and electroassisted deposition means. J. Microelectromech. Syst. 2008, 17, 1239–1253. [Google Scholar] [CrossRef]
- Accardo, A.; Courson, R.; Riesco, R.; Raimbault, V.; Malaquin, L. Direct laser fabrication of meso-scale 2D and 3D architectures with micrometric feature resolution. Addit. Manuf. 2018, 22, 440–446. [Google Scholar] [CrossRef]
- Maziz, A.; Courson, R.; Mesnilgrente, F.; Engel, E.; Leroy, L.; Sojic, N.; Malaquin, L.; Leichle, T. Rapid prototyping of a MEMS-based droplet dispenser using 3D printing. In Proceedings of the 45th International Conference on Micro and Nano Engineering (MNE 2019), Rhodes, Greece, 23–26 September 2019. [Google Scholar]
- Sandquist, M.; Wong, H.R. Biomarkers of sepsis and their potential value in diagnosis, prognosis and treatment. Expert Rev. Clin. Immunol. 2014, 10, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Wagner, T.A.; Gravett, C.A.; Healy, S.; Soma, V.; Patterson, J.C.; Gravett, M.G.; Rubens, C.E. Emerging biomarkers for the diagnosis of severe neonatal infections applicable to low resource settings. J. Glob. Health 2011, 1, 210–223. [Google Scholar] [PubMed]
- Meisner, A.; Kerr, K.F.; Thiessen-Philbrook, H.; Wilson, F.P.; Garg, A.X.; Shlipak, M.G.; Kavsak, P.; Whitlock, R.P.; Coca, S.G.; Parikh, C.R. Development of biomarker combinations for postoperative acute kidney injury via Bayesian model selection in a multicenter cohort study. Biomark. Res. 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmet, C.; Le Goff, G.C.; Brès, J.-C.; Rigal, D.; Blum, L.J.; Marquette, C.A. Multiplexed immunoassay for the rapid detection of anti-tumor-associated antigens antibodies. Analyst 2011, 136, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Madoz-Gurpide, J.; Misek, D.E.; Kuick, R.; Brenner, D.E.; Michailidis, G.; Haab, B.B.; Omenn, G.S.; Hanash, S. Development of natural protein microarrays for diagnosing cancer based on an antibody response to tumor antigens. J. Proteom. Res. 2004, 3, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Casiano, C.A.; Mediavilla-Varela, M.; Tan, E.M. Tumor-associated antigen arrays for the serological diagnosis of cancer. Mol. Cell Proteom. 2006, 5, 1745–1759. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Peng, B.; Lu, Y.; Xu, W.; Qian, W.; Zhang, J.-Y. Autoantibodies to tumor-associated antigens as biomarkers in cancer immunodiagnosis. Autoimmun. Rev. 2011, 10, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Odinsen, O.; Owusu-Ofori, S.; Dompreh, A.; Sarkodie, F.; Opare-Sem, O.; Parker, D.; Allain, J.-P. Antibody detection and kinetics of antibody production during early stages of immunization with Hepatitis B virus vaccine. Clin. Vaccine Immunol. 2007, 14, 1623–1628. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmet, C.; Vindas, K.; Alvarado Meza, R.; Garrigue, P.; Voci, S.; Sojic, N.; Maziz, A.; Courson, R.; Malaquin, L.; Leichle, T.; et al. Multiplexed Remote SPR Detection of Biological Interactions through Optical Fiber Bundles. Sensors 2020, 20, 511. https://doi.org/10.3390/s20020511
Desmet C, Vindas K, Alvarado Meza R, Garrigue P, Voci S, Sojic N, Maziz A, Courson R, Malaquin L, Leichle T, et al. Multiplexed Remote SPR Detection of Biological Interactions through Optical Fiber Bundles. Sensors. 2020; 20(2):511. https://doi.org/10.3390/s20020511
Chicago/Turabian StyleDesmet, Cloé, Karim Vindas, Ricardo Alvarado Meza, Patrick Garrigue, Silvia Voci, Neso Sojic, Ali Maziz, Rémi Courson, Laurent Malaquin, Thierry Leichle, and et al. 2020. "Multiplexed Remote SPR Detection of Biological Interactions through Optical Fiber Bundles" Sensors 20, no. 2: 511. https://doi.org/10.3390/s20020511