Abstract
Summary
Human cadaveric study has indicated that the metacarpal head (MCH) is intracapsular in location. We hypothesized that exposure to the intra-articular inflammatory milieu in psoriatic arthritis (PsA) will lead to bone loss in the MCH.
Introduction
To compare the bone structure and microstructure in the MCH between patients with PsA and healthy controls by high-resolution peripheral quantitative CT (HR-pQCT), and to ascertain factors associated with bone loss in PsA patients.
Methods
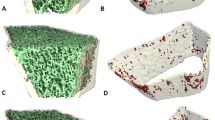
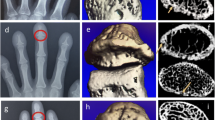
Sixty-two PsA patients without joint destruction and 62 age-, gender-, and body mass index–matched healthy subjects underwent HR-pQCT imaging of the second and third MCH (MCH 2&3). The number and volume of bone erosion and enthesiophytes, as well as volumetric bone mineral density (vBMD) and microstructure at the MCH 2&3, were recorded. Correlation analysis and multivariable linear regression models were used to determine the association of demographic and disease-specific variables with compromised bone structure and microstructure in PsA.
Results
At the MCH 2&3, bone erosion (p = 0.003) and enthesiophyte (p = 0.000) volumes in PsA patients were significantly larger than healthy controls. In PsA patients, older age was associated with a larger erosion and enthesiophyte volume. Concerning the mean vBMD and microstructure at the MCH 2&3, PsA patients had significantly lower mean vBMD (average vBMD − 6.9%, trabecular vBMD − 8.8%, peri-trabecular vBMD − 7.7%, meta-trabecular vBMD − 9.8%), trabecular bone volume fraction (− 8.8%), and trabecular thickness (− 8.1%) compared with control subjects. Multivariable regression analysis revealed that older age and a higher C-reactive protein level were associated with trabecular bone loss.
Conclusions
PsA patients had a higher burden of bone damages (erosions and enthesiophytes) and trabecular bone loss compared with healthy control at the MCH. Inflammation contributed to the deterioration in trabecular microstructure in these patients.


Similar content being viewed by others
References
Moll JM, Wright V (1973) Psoriatic arthritis. Semin Arthritis Rheum 3:55–78
Gladman DD, Antoni C, Mease P, Clegg DO, Nash P (2005) Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 64 Suppl 2:ii14–ii17
Ritchlin CT, Colbert RA, Gladman DD (2017) Psoriatic arthritis. N Engl J Med 376:957–970
Finzel S, Englbrecht M, Engelke K, Stach C, Schett G (2011) A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann Rheum Dis 70:122–127
Finzel S, Sahinbegovic E, Kocijan R, Engelke K, Englbrecht M, Schett G (2014) Inflammatory bone spur formation in psoriatic arthritis is different from bone spur formation in hand osteoarthritis. Arthritis Rheum 66:2968–2975
Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, de Jong J, van den Bergh J (2014) High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol 10:304–313
Simon D, Faustini F, Kleyer A, Haschka J, Englbrecht M, Kraus S, Hueber AJ, Kocijan R, Sticherling M, Schett G, Rech J (2016) Analysis of periarticular bone changes in patients with cutaneous psoriasis without associated psoriatic arthritis. Ann Rheum Dis 75:660–666
Simon D, Kleyer A, Faustini F et al (2018) Simultaneous quantification of bone erosions and enthesiophytes in the joints of patients with psoriasis or psoriatic arthritis - effects of age and disease duration. Arthritis Res Ther 20:203
Finzel S, Kraus S, Schmidt S, Hueber A, Rech J, Engelke K, Englbrecht M, Schett G (2013) Bone anabolic changes progress in psoriatic arthritis patients despite treatment with methotrexate or tumour necrosis factor inhibitors. Ann Rheum Dis 72:1176–1181
Kampylafka E, d’Oliveira I, Linz C et al (2018) Resolution of synovitis and arrest of catabolic and anabolic bone changes in patients with psoriatic arthritis by IL-17A blockade with secukinumab: results from the prospective PSARTROS study. Arthritis Res Ther 20:153
Simon D, Kleyer A, Stemmler F et al (2017) Age- and sex-dependent changes of intra-articular cortical and trabecular bone structure and the effects of rheumatoid arthritis. J Bone Miner Res 32:722–730
Zhu TY, Griffith JF, Qin L, Hung VW, Fong TN, Au SK, Kwok AW, Leung PC, Li EK, Tam LS (2015) Density, structure, and strength of the distal radius in patients with psoriatic arthritis: the role of inflammation and cardiovascular risk factors. Osteoporos Int 26:261–272
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, Group CS (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54:2665–2673
Stach CM, Bauerle M, Englbrecht M, Kronke G, Engelke K, Manger B, Schett G (2010) Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high-resolution computed tomography. Arthritis Rheum 62:330–339
Aletaha D, Alasti F, Smolen JS (2017) Disease activity states of the DAPSA, a psoriatic arthritis specific instrument, are valid against functional status and structural progression. Ann Rheum Dis 76:418–421
Scharmga A, Peters M, van den Bergh JP, Geusens P, Loeffen D, van Rietbergen B, Schoonbrood T, Vosse D, Weijers R, van Tubergen A (2018) Development of a scoring method to visually score cortical interruptions on high-resolution peripheral quantitative computed tomography in rheumatoid arthritis and healthy controls. PLoS One 13:e0200331
Scharmga A, Peters M, van Tubergen A, van den Bergh J, Barnabe C, Finzel S, van Rietbergen B, Geusens P (2016) Heterogeneity of cortical breaks in hand joints of patients with rheumatoid arthritis and healthy controls imaged by high-resolution peripheral quantitative computed tomography. J Rheumatol 43:1914–1920
Boutroy S, Chapurlat R, Vanden-Bossche A, Locrelle H, Thomas T, Marotte H (2015) Erosion or vascular channel? Arthritis Rheum 67:2956
Scharmga A, Keller KK, Peters M, van Tubergen A, van den Bergh JP, van Rietbergen B, Weijers R, Loeffen D, Hauge EM, Geusens P (2017) Vascular channels in metacarpophalangeal joints: a comparative histologic and high-resolution imaging study. Sci Rep 7:8966
Finzel S, Ohrndorf S, Englbrecht M, Stach C, Messerschmidt J, Schett G, Backhaus M (2011) A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum 63:1231–1236
Faustini F, Simon D, Oliveira I, Kleyer A, Haschka J, Englbrecht M, Cavalcante AR, Kraus S, Tabosa TP, Figueiredo C, Hueber AJ, Kocijan R, Cavallaro A, Schett G, Sticherling M, Rech J (2016) Subclinical joint inflammation in patients with psoriasis without concomitant psoriatic arthritis: a cross-sectional and longitudinal analysis. Ann Rheum Dis 75:2068–2074
Yushkevich PA, Yang G, Gerig G (2016) ITK-SNAP: an interactive tool for semi-automatic segmentation of multi-modality biomedical images. Conference Proc 2016:3342–3345
Yushkevich PA, Gerig G (2017) ITK-SNAP: an intractive medical image segmentation tool to meet the need for expert-guided segmentation of complex medical images. IEEE Pulse 8:54–57
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31:1116–1128
Vallaeys K, Kacem A, Legoux H, Le Tenier M, Hamitouche C, Arbab-Chirani R (2015) 3D dento-maxillary osteolytic lesion and active contour segmentation pilot study in CBCT: semi-automatic vs manual methods. Dento Maxillo Facial Radiol 44:20150079
Besson FL, Henry T, Meyer C et al (2018) Rapid contour-based segmentation for (18)F-FDG PET imaging of lung tumors by using ITK-SNAP: comparison to expert-based segmentation. Radiology 288:277–284
Akudjedu TN, Nabulsi L, Makelyte M et al (2018) A comparative study of segmentation techniques for the quantification of brain subcortical volume. Brain Imaging Behav 12:1678–1695
Lu P, Barazzetti L, Chandran V, Gavaghan K, Weber S, Gerber N, Reyes M (2018) Highly accurate facial nerve segmentation refinement from CBCT/CT imaging using a super-resolution classification approach. IEEE Trans Biomed Eng 65:178–188
Lories RJ, Schett G (2012) Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin N Am 38:555–567
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515
Yang H, Yu A, Burghardt AJ, Virayavanich W, Link TM, Imboden JB, Li X (2017) Quantitative characterization of metacarpal and radial bone in rheumatoid arthritis using high resolution-peripheral quantitative computed tomography. Int J Rheum Dis 20:353–362
Zhu TY, Griffith JF, Qin L et al (2013) Structure and strength of the distal radius in female patients with rheumatoid arthritis: a case-control study. J Bone Miner Res 28:794–806
Simon D, Kleyer A, Englbrecht M, Stemmler F, Simon C, Berlin A, Kocijan R, Haschka J, Hirschmann S, Atreya R, Neurath MF, Sticherling M, Rech J, Hueber AJ, Engelke K, Schett G (2018) A comparative analysis of articular bone in large cohort of patients with chronic inflammatory diseases of the joints, the gut and the skin. Bone 116:87–93
Zhu TY, Griffith JF, Qin L, Hung VW, Fong TN, Kwok AW, Leung PC, Li EK, Tam LS (2012) Bone density and microarchitecture: relationship between hand, peripheral, and axial skeletal sites assessed by HR-pQCT and DXA in rheumatoid arthritis. Calcif Tissue Int 91:343–355
Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, Araujo E, Hueber AJ, Harre U, Engelke K, Schett G (2014) Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis 73:854–860
Fouque-Aubert A, Boutroy S, Marotte H, Vilayphiou N, Bacchetta J, Miossec P, Delmas PD, Chapurlat RD (2010) Assessment of hand bone loss in rheumatoid arthritis by high-resolution peripheral quantitative CT. Ann Rheum Dis 69:1671–1676
Acknowledgments
We thank all patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval (CRE-2012.082) was obtained from the Ethics Committee of The Chinese University of Hong Kong-New Territories East Cluster Hospitals with written informed consent obtained from all participants.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1993 kb)
Rights and permissions
About this article
Cite this article
Wu, D., Griffith, J., Lam, S. et al. Comparison of bone structure and microstructure in the metacarpal heads between patients with psoriatic arthritis and healthy controls: an HR-pQCT study. Osteoporos Int 31, 941–950 (2020). https://doi.org/10.1007/s00198-020-05298-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05298-z