Abstract
Purpose
To assess the safety, biodistribution, and radiation dosimetry of the novel positron emission tomography (PET) radiopharmaceutical 1-((2-fluoro-6-[[18F]]fluorophenyl)sulfonyl)-4-((4-methoxyphenyl)sulfonyl)piperazine ([18F]DASA-23) in healthy volunteers.
Methods
We recruited 5 healthy volunteers who provided a written informed consent. Volunteers were injected with 295.0 ± 8.2 MBq of [18F]DASA-23 intravenously. Immediately following injection, a dynamic scan of the brain was acquired for 15 min. This was followed by serial whole-body PET/MRI scans acquired up to 3 h post-injection. Blood samples were collected at regular intervals, and vital signs monitored pre- and post-radiotracer administration. Regions of interest were drawn around multiple organs, time-activity curves were calculated, and organ uptake and dosimetry were estimated with OLINDA/EXM (version 1.1) software.
Results
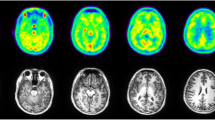
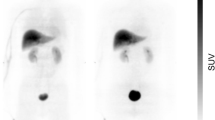
All subjects tolerated the PET/MRI examination, without adverse reactions to [18F]DASA-23. [18F]DASA-23 passively crossed the blood-brain barrier, followed by rapid clearance from the brain. High accumulation of [18F]DASA-23 was noted in organs such as the gallbladder, liver, small intestine, and urinary bladder, suggesting hepatobiliary and urinary clearance. The effective dose of [18F]DASA-23 was 23.5 ± 5.8 μSv/MBq.
Conclusion
We successfully completed a pilot first-in-human study of [18F]DASA-23. Our results indicate that [18F]DASA-23 can be used safely in humans to evaluate pyruvate kinase M2 levels. Ongoing studies are evaluating the ability of [18F]DASA-23 to visualize intracranial malignancies, NCT03539731.
Trial registration
ClinicalTrials.gov, NCT03539731 (registered 28 May 2018)




Similar content being viewed by others
References
Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro-Oncology. 2017;19:v1–v88.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Patronas NJ, Di Chiro G, Brooks RA, DeLaPaz RL, Kornblith PL, Smith BH, et al. Work in progress: [18F]fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology. 1982;144:885–9.
Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–11.
Olivero WC, Dulebohn SC, Lister JR. The use of PET in evaluating patients with primary brain tumours: is it useful. J Neurol Neurosurg Psychiatry. 1995;58:250–2.
Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96:191–7.
Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography. AJNR Am J Neuroradiol. 1998;19:407–13.
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40:205–12.
Weber WA, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med. 2000;27:542–9.
Kläsner B, Buchmann N, Gempt J, Ringel F, Lapa C, Krause BJ. Early [18F]FET-PET in gliomas after surgical resection: comparison with MRI and histopathology. PLoS One. 2015;10:e0141153.
Juhasz C, Dwivedi S, Kamson DO, Michelhaugh SK, Mittal S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol Imaging. 2014;13.
Beinat C, Alam IS, James ML, Srinivasan A, Gambhir SS. Development of [(18)F]DASA-23 for imaging tumor glycolysis through noninvasive measurement of pyruvate kinase M2. Mol Imaging Biol. 2017;19:665–72.
Beinat C, Patel CB, Xie Y, Gambhir SS. Evaluation of glycolytic response to multiple classes of anti-glioblastoma drugs by noninvasive measurement of pyruvate kinase M2 using [18F]DASA-23. Mol Imaging Biol. 2019.
Beinat C, Haywood T, Chen YS, Patel CB, Alam IS, Murty S, et al. The utility of [18F]DASA-23 for molecular imaging of prostate cancer with positron emission tomography. Mol Imaging Biol. 2018;20:1015–24.
Haywood T, Beinat C, Gowrishankar G, Patel CB, Alam IS, Murty S, et al. Positron emission tomography reporter gene strategy for use in the central nervous system. Proc Natl Acad Sci U S A. 2019;116:11402.
Agnihotri S, Zadeh G. Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro-Oncology. 2016;18:160–72.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3.
Witney TH, James ML, Shen B, Chang E, Pohling C, Arksey N, et al. PET imaging of tumor glycolysis downstream of hexokinase through noninvasive measurement of pyruvate kinase M2. Sci Transl Med. 2015;7:310ra169.
Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–47.
Mukherjee J, Phillips JJ, Zheng S, Wiencke J, Ronen SM, Pieper RO. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS One. 2013;8:e57610.
Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, et al. Zero TE MR bone imaging in the head. Magn Reson Med. 2016;75:107–14.
Delso G, Wiesinger F, Sacolick LI, Kaushik SS, Shanbhag DD, Hullner M, et al. Clinical evaluation of zero-echo-time MR imaging for the segmentation of the skull. J Nucl Med. 2015;56:417–22.
Recommendations of the International Commission on Radiological Protection. Annals of the ICRP.1990 1991;21:1–201.
Hsiao IT, Lin KJ, Huang KL, Huang CC, Chen HS, Wey SP, et al. Biodistribution and radiation dosimetry for the tau tracer (18)F-THK-5351 in healthy human subjects. J Nucl Med. 2017;58:1498–503.
Harada R, Okamura N, Furumoto S, Furukawa K, Ishiki A, Tomita N, et al. 18F-THK5351: a novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J Nucl Med. 2016;57:208–14.
Senda M, Brooks DJ, Farrar G, Somer EJ, Paterson CL, Sasaki M, et al. The clinical safety, biodistribution and internal radiation dosimetry of flutemetamol (18F) injection in healthy Japanese adult volunteers. Ann Nucl Med. 2015;29:627–35.
Beauregard J-M, Croteau É, Ahmed N, van Lier JE, Bénard F. Assessment of human biodistribution and dosimetry of 4-fluoro-11β-methoxy-16α-18F-fluoroestradiol using serial whole-body PET/CT. J Nucl Med. 2009;50:100–7.
Paquette M, Lavallée É, Phoenix S, Ouellet R, Senta H, van Lier JE, et al. Improved estrogen receptor assessment by PET using the novel radiotracer 18F-4FMFES in estrogen receptor–positive breast cancer patients: an ongoing phase II clinical trial. J Nucl Med. 2018;59:197–203.
Douglas BR, Jansen JB, Tham RT, Lamers CB. Coffee stimulation of cholecystokinin release and gallbladder contraction in humans. Am J Clin Nutr. 1990;52:553–6.
Marciani L, Cox EF, Hoad CL, Totman JJ, Costigan C, Singh G, et al. Effects of various food ingredients on gall bladder emptying. Eur J Clin Nutr. 2013;67:1182–7.
Mittra ES, Goris ML, Iagaru AH, Kardan A, Burton L, Berganos R, et al. Pilot pharmacokinetic and dosimetric studies of 18F-FPPRGD2: a PET radiopharmaceutical agent for imaging αvβ3 integrin levels. Radiology. 2011;260:182–91.
Ronald JA, Kim B-S, Gowrishankar G, Namavari M, Alam IS, Souza A, et al. A PET imaging strategy to visualize activated T cells in acute graft-versus-host disease elicited by allogenic hematopoietic cell transplant. Cancer Res. 2017;77:2893.
Hjørnevik T, Cipriano PW, Shen B, Park JH, Gulaka P, Holley D, et al. Biodistribution and radiation dosimetry of 18F-FTC-146 in humans. J Nucl Med. 2017;58:2004–9.
Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). Annals of the ICRP. 1998;28:1–126.
Kaushik A, Jaimini A, Tripathi M, D'Souza M, Sharma R, Mondal A, et al. Estimation of radiation dose to patients from (18) FDG whole body PET/CT investigations using dynamic PET scan protocol. Indian J Med Res. 2015;142:721–31.
Sandström M, Velikyan I, Garske-Román U, Sörensen J, Eriksson B, Granberg D, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54:1755–9.
McParland BJ, Wall A, Johansson S, Sorensen J. The clinical safety, biodistribution and internal radiation dosimetry of [18F]fluciclovine in healthy adult volunteers. Eur J Nucl Med Mol Imaging. 2013;40:1256–64.
Bezrukov I, Schmidt H, Mantlik F, Schwenzer N, Brendle C, Schölkopf B, et al. MR-based attenuation correction methods for improved PET quantification in lesions within bone and susceptibility artifact regions. J Nucl Med. 2013;54:1768–74.
Iagaru A, Mittra E, Minamimoto R, Jamali M, Levin C, Quon A, et al. Simultaneous whole-body time-of-flight 18F-FDG PET/MRI: a pilot study comparing SUVmax with PET/CT and assessment of MR image quality. Clin Nucl Med. 2015;40:1–8.
Acknowledgments
We thank the Cyclotron and Radiochemistry Facility at Stanford for their support in particular Dr. Fred Chin, Jun Hyung Park, Jessa B. Castillo, and Carmen Azevedo. We would also like to thank Geoffrey Warnock from PMOD Technologies LLC and Stanford Division of Neuro-oncology in particular Drs. Lawrence Recht, Seema Nagpal, Reena Thomas, Priya Yerraballa, Sophie Bertrand, and Mark Santos. CB acknowledges receipt of a Stanford Translational Research and Applied Medicine fellowship.
Funding
This work was supported by The Ben and Catherine Ivy Foundation (SSG), GE Healthcare (SSG), and Stanford Translational Research and Applied Medicine Fellowship (CB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Dosimetry
Electronic supplementary material
ESM 1
(DOCX 77 kb)
Rights and permissions
About this article
Cite this article
Beinat, C., Patel, C.B., Haywood, T. et al. Human biodistribution and radiation dosimetry of [18F]DASA-23, a PET probe targeting pyruvate kinase M2. Eur J Nucl Med Mol Imaging 47, 2123–2130 (2020). https://doi.org/10.1007/s00259-020-04687-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04687-0