Abstract
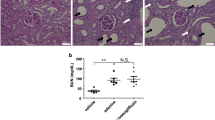
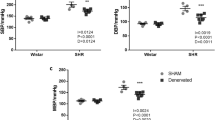
We recently reported that a 4% high-salt diet + saline for drinking (HS + saline) leads to a catabolic state, reduced heart rate, and suppression of cardiovascular energy expenditure in mice. We suggested that HS + saline reduces heart rate via the suppression of the sympathetic nervous system to compensate for the high salt intake-induced catabolic state. To test this hypothesis, we directly measured renal sympathetic nerve activity (RSNA) in conscious Sprague-Dawley (SD) rats using a radiotelemetry system. We confirmed that HS + saline induced a catabolic state. HS + saline decreased heart rate, while also reducing RSNA in SD rats. In contrast, Dahl salt-sensitive (DSS) rats exhibited no change in heart rate and increased RSNA during high salt intake. Renal denervation significantly decreased heart rate and attenuated the catabolic state independent of blood pressure in DSS rats fed HS + saline, suggesting that salt-sensitive animals were unable to decrease cardiovascular energy consumption due to abnormal renal sympathetic nerve activation during high salt intake. These findings support the hypothesis that RSNA mediates heart rate during high salt intake in SD rats. However, the insensitivity of heart rate and enhanced RSNA observed in DSS rats may be additional critical diagnostic factors for salt-sensitive hypertension. Renal denervation may benefit salt-sensitive hypertension by reducing its effects on catabolism and cardiovascular energy expenditure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bonventre JV, Leaf A. Sodium homeostasis: steady states without a set point. Kidney Int. 1982;21:880–3.
Hollenberg NK. Set point for sodium homeostasis: surfeit, deficit, and their implications. Kidney Int. 1980;17:423–9.
Rakova N, Kitada K, Lerchl K, Dahlmann A, Birukov A, Daub S, et al. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Investig. 2017;127:1932–43.
Lerchl K, Rakova N, Dahlmann A, Rauh M, Goller U, Basner M, et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension. 2015;66:850–7.
Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, Kopp C, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–31.
Kitada K, Daub S, Zhang Y, Klein JD, Nakano D, Pedchenko T, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Investig. 2017;127:1944–59.
Boudoulas KD, Borer JS, Boudoulas H. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology. 2015;132:199–212.
Palatini P. Role of elevated heart rate in the development of cardiovascular disease in hypertension. Hypertension. 2011;58:745–50.
Araujo JP, Lourenco P, Rocha-Goncalves F, Ferreira A, Bettencourt P. Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol. 2011;146:359–63.
Loncar G, Fulster S, von Haehling S, Popovic V. Metabolism and the heart: an overview of muscle, fat, and bone metabolism in heart failure. Int J Cardiol. 2013;162:77–85.
de Lucia C, Piedepalumbo M, Paolisso G, Koch WJ. Sympathetic nervous system in age-related cardiovascular dysfunction: pathophysiology and therapeutic perspective. Int J Biochem Cell Biol. 2019;108:29–33.
Sufiun A, Rafiq K, Fujisawa Y, Rahman A, Mori H, Nakano D, et al. Effect of dipeptidyl peptidase-4 inhibition on circadian blood pressure during the development of salt-dependent hypertension in rats. Hypertens Res. 2015;38:237–43.
Rafiq K, Fujisawa Y, Sherajee SJ, Rahman A, Sufiun A, Kobori H, et al. Role of the renal sympathetic nerve in renal glucose metabolism during the development of type 2 diabetes in rats. Diabetologia. 2015;58:2885–98.
Lei B, Nakano D, Fujisawa Y, Liu Y, Hitomi H, Kobori H, et al. N-type calcium channel inhibition with cilnidipine elicits glomerular podocyte protection independent of sympathetic nerve inhibition. J Pharm Sci. 2012;119:359–67.
Luippold G, Beilharz M, Muhlbauer B. Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transpl. 2004;19:342–7.
Salman IM, Sarma Kandukuri D, Harrison JL, Hildreth CM, Phillips JK. Direct conscious telemetry recordings demonstrate increased renal sympathetic nerve activity in rats with chronic kidney disease. Front Physiol. 2015;6:218.
Stocker SD, Muntzel MS. Recording sympathetic nerve activity chronically in rats: surgery techniques, assessment of nerve activity, and quantification. Am J Physiol Heart Circ Physiol. 2013;305:H1407–16.
Nagasu H, Satoh M, Fujimoto S, Tomita N, Sasaki T, Kashihara N. Azelnidipine attenuates glomerular damage in Dahl salt-sensitive rats by suppressing sympathetic nerve activity. Hypertens Res. 2012;35:348–55.
Cohn JN. Sympathetic nervous system activity and the heart. Am J Hypertens. 1989;2:353S–6S.
Thames MD, Miller BD, Abboud FM. Baroreflex regulation of renal nerve activity during volume expansion. Am J Physiol. 1982;243:H810–14.
Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol. 2015;308:R112–22.
Schlaich MP, Esler MD, Fink GD, Osborn JW, Euler DE. Targeting the sympathetic nervous system: critical issues in patient selection, efficacy, and safety of renal denervation. Hypertension. 2014;63:426–32.
Prosnitz EH, DiBona GF. Effect of decreased renal sympathetic nerve activity on renal tubular sodium reabsorption. Am J Physiol. 1978;235:F557–63.
DiBona GF. Neural regulation of renal tubular sodium reabsorption and renin secretion. Fed Proc. 1985;44:2816–22.
DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197.
Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–13.
Chen W, Chang Y, He L, Jian X, Li L, Gao L, et al. Effect of renal sympathetic denervation on hepatic glucose metabolism and blood pressure in a rat model of insulin resistance. J Hypertens. 2016;34:2465–74.
Tsioufis C, Dimitriadis K, Kasiakogias A, Kalos T, Liatakis I, Koutra E, et al. Effects of multielectrode renal denervation on elevated sympathetic nerve activity and insulin resistance in metabolic syndrome. J Hypertens. 2017;35:1100–8.
Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–55.
Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–45.
Foss JD, Fiege J, Shimizu Y, Collister JP, Mayerhofer T, Wood L, et al. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol Rep. 2018;6:e13602.
Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, et al. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transpl. 2010;25:2889–98.
Tudorancea I, Lohmeier TE, Alexander BT, Pieptu D, Serban DN, Iliescu R. Reduced renal mass, salt-sensitive hypertension is resistant to renal denervation. Front Physiol. 2018;9:455.
Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–9.
Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401.
Kario K, Ogawa H, Okumura K, Okura T, Saito S, Ueno T, et al. SYMPLICITY HTN-Japan—first randomized controlled trial of catheter-based renal denervation in asian patients. Circ J. 2015;79:1222–9.
Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163–70.
Feyz L, Theuns DA, Bhagwandien R, Strachinaru M, Kardys I, Van Mieghem NM. et al. Atrial fibrillation reduction by renal sympathetic denervation: 12 months’ results of the AFFORD study. Clin Res Cardiol. 2018;10:018-1391
Warchol-Celinska E, Prejbisz A, Kadziela J, Florczak E, Januszewicz M, Michalowska I, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept Phase II trial. Hypertension. 2018;72:381–90.
Krawczyk-Ozog A, Tokarek T, Moczala K, Siudak Z, Dziewierz A, Mielecki W, et al. Long-term quality of life and clinical outcomes in patients with resistant hypertension treated with renal denervation. Postepy Kardiol Interwencyjnej. 2016;12:329–33.
Kordalis A, Tsiachris D, Pietri P, Tsioufis C, Stefanadis C. Regression of organ damage following renal denervation in resistant hypertension: a meta-analysis. J Hypertens. 2018;36:1614–21.
Watanabe H, Iwanaga Y, Miyaji Y, Yamamoto H, Miyazaki S. Renal denervation mitigates cardiac remodeling and renal damage in Dahl rats: a comparison with beta-receptor blockade. Hypertens Res. 2016;39:217–26.
Jiang W, Tan L, Guo Y, Li X, Tang X, Yang K. Effect of renal denervation procedure on left ventricular hypertrophy of hypertensive rats and its mechanisms. Acta Cir Bras. 2012;27:815–20.
Bohm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, et al. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J. 2019;40:743–51.
Acknowledgements
This study was partially supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI: 18H03191) and the Salt Sciences Foundation (18C5) to AN. We are deeply grateful to Mr. Masao Masui and Ms. Sachiko Okegawa (Osaka General City Hospital) for animal maintenance at Osaka General City Hospital.
Author information
Authors and Affiliations
Contributions
NM, KK, YF, DY, LL, YZ, and TM performed the animal experiments. KK, TM, YK, TY, JT, and AN provided essential material and contributed to the design of the experiments. SK measured, analyzed, and interpreted the tissue noradrenaline content data. NM and YF performed animal surgery and radiotelemetry measurements. NM, KK, and YF analyzed the radiotelemetry data. NM, KK, DN, DY, JT, and AN designed and planned the experiments and analyzed and interpreted the data. NM, KK, DN, FL, JT, and AN wrote the paper. YK, TY, FL, JT, and AN supervised the research project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Morisawa, N., Kitada, K., Fujisawa, Y. et al. Renal sympathetic nerve activity regulates cardiovascular energy expenditure in rats fed high salt. Hypertens Res 43, 482–491 (2020). https://doi.org/10.1038/s41440-019-0389-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0389-1
Keywords
This article is cited by
-
Comparison of the effects of renal denervation at early or advanced stages of hypertension on cardiac, renal, and adipose tissue pathology in Dahl salt-sensitive rats
Hypertension Research (2024)
-
Do tissue sodium levels support renal denervation?
Hypertension Research (2024)
-
Dietary salt intake predicts future development of metabolic syndrome in the general population
Hypertension Research (2023)
-
Contributions of renal water loss and skin water conservation to blood pressure elevation in spontaneously hypertensive rats
Hypertension Research (2023)
-
Annual reports on hypertension research 2020
Hypertension Research (2022)