Abstract
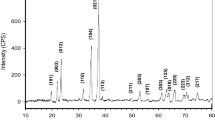
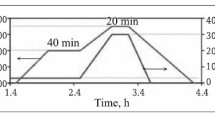
The method of calciothermic reduction of B4C was proposed for preparing CaB6. The phase transition and morphology evolution during the reaction were investigated in detail. The experimental results reveal that Ca first reacts with B4C to generate CaB2C2 and CaB6 at a low temperature and that the CaB2C2 subsequently reacts with Ca to produce CaB6 and CaC2 at a high temperature. After the products were leached to remove the byproduct CaC2, pure CaB6 was obtained. The grain size of the prepared CaB6 was 2–3 μm, whereas its particle size was 4–13 μm; it inherited the particle size of B4C. The residual C content of the product was decreased to 1.03wt% after the first reaction at 1173 K for 4 h and the second reaction at 1623 K for 4 h.
Similar content being viewed by others
References
Nisso Tsushinsha, ed, Handbook on High-melting-point Composites, Nisso Tsushinsha, Ehime, 1977.
R.A. Cutler, Engineered Materials Handbook, Vol. 4, S.J. Schneider Jr. ed., ASM International, The Materials Information Society, Metals Park, Ohio, 1991, p. 787.
P.A. Dearnley and T. Bell, Engineering the surface with boron based materials, Surf. Eng, 1(1985), No. 3, p. 203.
H.J. Tromp, P. Van Gelderen, P.J. Kelly, G. Brocks, and P.A. Bobbert, CaB6: a new semiconducting material for spin electronics, Phys. Rev. Lett., 87(2001), art. No. 016401.
D.P. Young, D. Hall, M.E. Torelli, Z. Fisk, J.L. Sarrao, J.D. Thompson, H.R. Ott, S.B. Oseroff, R.G. Goodrich, and R. Zysler, High-temperature weak ferromagnetism in a low-density free-electron gas, Nature, 397(1999), p. 412.
L.H. Bao, X.P. Qi, L.M. Chao, and O. Tegus, Synthesis, and magnetic and optical properties of nanocrystalline alkaline-earth hexaborides, CrystEngComm, 18(2016), p. 1223.
H. Razavi-Zadeh and S. Mirdamadi.T, Deoxidizing copper with CaB6, JOM, 39(1987), No. 2, p. 42.
K.G. Schmitt-Thomas, A. Lipp, and K. Schwetz, Process for the Production of Oxygen-Free Copper Casting and Moldings, United States Patent, Appl. 738819, 1976.
T. Rymon-Lipinski, B. Schmelzer, and S. Ulitzka, Tests on the oxidation-inhibiting effect of CaB6 in refractory MgO-C materials, Steel Res., 65(1994), No. 6, p. 234.
J.W. Butler, Neutron-absorbing bricks made from CaB6, Nucl. Instrum. Methods, 7(1960), No. 2, p. 201.
M.T. Zheng, H.W. Dong, Y. Xiao, S.T. Liu, H. Hu, Y.R. Liang, L.Y. Sun, and Y.L. Liu, Facile one-step and high-yield synthesis of few-layered and hierarchically porous boron nitride nanosheets, RSC Adv., 6(2016), No. 51, p. 45402.
B.A. Galanov, V.V. Kartuzov, O.N. Grigoriev, L.M. Melakh, S.M. Ivanov, E.V. Kartuzov, and P. Swoboda, Penetration resistance of B4C-CaB6 based light-weight armor materials, Procedia Eng., 58(2013), p. 328.
P. Seenuvasaperumal, A. Elayaperumal, and R. Jayavel, Influence of calcium hexaboride reinforced magnesium composite for the mechanical and tribological behviour, Tribol. Int., 111(2017), p. 18.
Y. Gao, Z.D. Liu, Q. Wang, and Y.T. Wang, Mcrostructure and mechanical properties of Nb-Mo-ZrB2 composites prepared by hot-pressing sintering, Int. J. Miner. Metall. Mater, 25(2018), No. 7, p. 824.
Y.R. Zhang, Q. Cai, Y.C. Liu, Z.Q. Ma, C. Li, and H.J. Li, Evaluation of precipitation hardening in TiC-reinforced Ti2AlNb-based alloys, Int. J. Miner. Metall. Mater, 25 (2018), No. 4, p. 453.
L. Zhang, G.H. Mn, and H.S. Yu, Reaction mechanism and size control of CaB6 micron powder synthesized by the boroncarbide method, Ceram. Int., 35(2009), No. 8, p. 3533.
M. Kakiage, S. Shiomi, I. Yanase, and H. Kobayashi, Low-temperature synthesis of calcium hexaboride powder via transient boron carbide formation, J. Am. Ceram. Soc, 98 (2015), No. 9, p. 2724.
D. Yilmaz, U. Savaci, N. Koç, and S. Turanb, Carbothermic reduction synthesis of calcium hexaboride using PVA-calcium hexaborate mixed gels, Ceram. Int., 44(2018), No. 3, p. 2976.
M. Kakiage, S. Shiomi, T. Ohashi, and H. Kobayashi, Effect of calcium carbonate particle size on formation and morphology of calcium hexaboride powder synthesized from condensed boric acid-poly (vinyl alcohol) product, Adv. Powder Technol, 29(2018), No. 1, p. 36.
K. Bao, L.X. Lin, H. Chang, and S.W. Zhang, Low-temperature synthesis of calcium hexaboride nanoparticles via magnesiothermic reduction in molten salt, J. Ceram. Soc. Jpn., 125(2017), No. 12, p. 866.
Ö. Balcı, D. Ağaoğulları, İ. Duman, and M.L. Öveçoğlu, Synthesis of CaB6 powders via mechanochemical reaction of Ca/B2O3 blends, Powder Technol, 225(2012), p. 136.
X. Huang, J.C. Zhong, L.S. Dou, and K. Wang, Combustion synthesis of CaB6 powder from calcium hexaborate and Mg, Int. J. Refract. Met. Hard Mater, 28(2010), No. 2, p. 143.
X. Wang and Y.C. Zhai, An electrochemical method for the preparation of CaB6 crystal powder, J. Appl. Electrochem., 39(2009), p. 1797.
W. Weng, M.Y. Wang, X.Z. Gong, Z. Wang, D. Wang, and Z.C. Guo, Electrochemical conversions of soluble borates to CaB6 with superior optical property in NaCl-CaCl2 melt, J. Electrochem. Soc, 165(2018), No. 10, p. E477.
S. Angappan, M. Helan, A. Visuvasam, L.J. Berchmans, and V. Ananth, Electrolytic preparation of CaB6 by molten salt technique, Ionics, 17(2011), No. 6, p. 527.
S.Q. Zheng, G.H. Min, Z.D. Zou, H.S. Yu, and J.D. Han, Synthesis of calcium hexaboride powder via the reaction of calcium carbonate with boron carbide and carbon, J. Am. Ceram. Soc, 84(2001), No. 11, p. 2725.
B. Albert and K. Schmitt, CaB2C2: Reinvestigation of a semiconducting boride carbide with a layered structure and an interesting boron/carbon ordering scheme, Inorg. Chem., 38(1999), No. 26, p. 6159.
J. Akimitsu, K. Takenawa, K. Suzuki, H. Harima, and Y. Kuramoto, High-temperature ferromagnetism in CaB2C2, Science, 293(2001), No. 5532, p. 1125.
A.V. Blinder, S.P. Gordienko, É.V. Marek, and V.B. Muratov, Thermodynamic properties of calcium hexaboride, Powder Metall. Met. Ceram., 36(1997), No. 7–8, p. 409.
W. Ostwald, Über die vermeintliche Isomerie des roten und gelben Quecksilberoxyds und die Oberflächenspannung fester Körper, Z. Phys. Chem., 34(1900), p. 495.
L. Zhang, G.H. Min, H.S. Yu, H.M. Chen, and G. Feng, The size and morphology of fine CaB6 powder synthesized by nanometer CaCO3 as reactant, Key Eng. Mater, 326–328(2006), p. 369.
S.S.N. Murthy, M. Patel, J.J. Reddy, and V.V. Bhanu Prasad, Influence of B4C particle size on the synthesis of ZrB2 by boro/carbothermal reduction method, Trans. Indian Inst. Met, 71(2018), No. 1, p. 57.
Acknowledgement
This work was financially supported by the Fundamental Research Funds for the Central Universities of China (No. FRF-GF-17-B41).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, Gh., Wu, Yd. et al. Preparation of CaB6 powder via calciothermic reduction of boron carbide. Int J Miner Metall Mater 27, 37–45 (2020). https://doi.org/10.1007/s12613-019-1873-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1873-y