Abstract
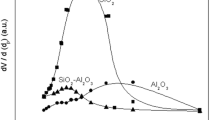
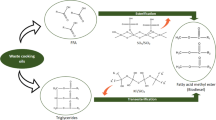
In this work, different magnesium silicate mineral samples based on antigorite, lizardite, chrysotile (which have the same general formula Mg3Si2O5(OH)4), and talc (Mg3Si4O10(OH)2) were reacted with KOH to prepare catalysts for biodiesel production. Simple impregnation with 20wt% K and treatment at 700-900°C led to a solid-state reaction to mainly form the K2MgSi04 phase in all samples. These results indicate that the K ion can diffuse into the different Mg silicate structures and textures, likely through intercalation in the interlayer space of the different mineral samples followed by dehydroxylation and K2MgSi04 formation. All the materials showed catalytic activity for the transesterification of soybean oil (1:6 of oil: methanol molar ratio, 5wt% of catalyst, 60°C). However, the best results were obtained for the antigorite and chrysotile precursors, which are discussed in terms of mineral structure and the more efficient formation of the active phase K2MgSi04.
Similar content being viewed by others
References
A.L. Auzende, I. Daniel, B. Reynard, C. Lemaire, and F. Guyot, High-pressure behavior of serpentine minerals: A Raman spectroscopic study, Phys. Chem. Miner., 31(2004), No. 5, p. 269.
S. Guillot, S. Schwartz, B. Reynard, P. Agard, and C. Prigent, Tectonic significance of serpentinites, Tectonophysics, 646(2015), p. 1.
B.W. Evans, K. Hattori, and A. Baronnet, Serpentinite: what, why, where? Elements, 9(2013), No. 2, p. 99.
B.T. Mossman, J. Bignon, M. Corn, A. Seaton, and J.B. Gee, Asbestos: scientific developments and implications for public policy, Science, 247(1990), No. 4940, p. 294.
G.C. Capitani and M. Mellini, The crystal structure of a second antigorite polysome (m = 16), by single-crystal synchrotron diffraction, Am. Mineral., 91(2006), No. 2–3, p. 394.
M. Claverie, A. Dumas, C. Careme, M. Poirier, C. Le Roux, P. Micoud, F. Martin, and C. Aymonier, Synthetic talc and talc-like structures: Preparation, features and applications, Chemistry, 24(2018), No. 3, p. 519.
S.S. Vieira, G.M. Paz, A.P.C. Teixeira, E.M. Moura, O.R. Carmignano, R.C.O. Sebastiao, and R.M. Lago, Solid state reaction of serpentinite Mg3Si2O5(OH)4 with Li+ to produce Li4SiO4/MgO composites for the efficient capture of CO2, J. Environ. Chem. Eng., 6(2018), No. 4, p. 4189.
G.M. Paz, S.S. Vieira, A.C. Bertoli, F.C. Ballotin, E.M. de Moura, A.P.C. Teixeira, D. Costa, O. Carmignano, and R.M. Lago, Solid state reaction of serpentinite Mg3Si2O5(OH)4 with NaOH to produce a new basic catalytic phase Na2Mg2Si2O7 for biodiesel production, J. Braz. Chem. Soc, 29(2018), No. 9, p. 1823.
F.C. Ballotin, T.E. Cibaka, T.A. Ribeiro-Santos, E.M. Santos, AP. de Carvalho Teixeira, and R.M. Lago, K2MgSiO4: A novel K+-trapped biodiesel heterogeneous catalyst produced from serpentinite Mg3Si2O5(OH)4, J. Mol. Catal. A: Chem., 422(2016), p. 258.
U. Schuchardt, R. Sercheli, and R.M. Vargas, Transesterification of vegetable oils: A review, J. Braz. Chem. Soc, 9(1998), No. 3, p. 199.
A.P.C. Teixeira, E.M. Santos, A.F.P. Vieira, and R.M. Lago, Use of chrysotile to produce highly dispersed K-doped MgO catalyst for biodiesel synthesis, Chem. Eng. J., 232(2013), p. 104.
A. Shakoor and N.L. Thomas, Talc as a nucleating agent and reinforcing filler in poly(lactic acid) composites, Pofym. Eng. Sci., 54(2014), No. 1, p. 64.
R.G. Coleman, Petrologic and geophysical nature of serpentinites, Geol. Soc. Am. Bull, 82(1971), No. 4, p. 897.
M. Wesolowski, Thermal decomposition of talc: A review, Thermochim. Acta, 78(1984), No. 1–3, p. 395.
M.D. Menzel, C.J. Garrido, V.L. Sanchez-Vizcaino, C. Marchesi, K. Hidas, M.P. Escayola, and A.D. Huertas, Carbonation of mantle peridotite by CO2-rich fluids: The formation of listvenites in the Advocate ophiolite complex (Newfoundland, Canada), Lithos, 323(2018), p. 238.
X. Liu, X. Liu, and Y. Hu, Investigation of the thermal decomposition of talc, Clays Clay Miner., 62(2014), No. 2, p. 137.
C. Viti, Serpentine minerals discrimination by thermal analysis, Am. Mineral, 95(2010), No. 4, p. 631.
H. Maleki, M. Kazemeini, and F. Bastan, Transesterification of canola oil to biodiesel using CaO/Talc nanopowder as a mixed oxide catalyst, Chem. Eng. Technol, 40(2017), No. 10, p. 1923.
A.F. Gualtieri, N.B. Gandolfi, S. Pollastri, M. Burghammer, E. Tibaldi, F. Belpoggi, K. Pollok, F. Langenhorst, R. Vigliaturo, and G. Drazic, New insights into the toxicity of mineral fibers: A combined in situ synchrotron μ-XRD and HR-TEM study of chrysotile, crocidolite, and erionite fibers found in the tissues of Sprague-Dawley rats, Toxicol. Lett, 274(2017), p. 20.
CM. Yarborough, The risk of mesothelioma from exposure to chrysotile asbestos, Curr. Opin. Pulm. Med., 13(2007), No. 4, p. 334.
B. Ersoy, S. Dikmen, A. Yildiz, R. Gören, and Ö. Elitok, Mineralogical and physicochemical properties of talc from Emirdağ, Afyonkarahisar, Turk. J. Earth Sci., 22(2013), No. 4, p. 632.
Acknowledgements
The authors acknowledge Pedras Congonhas LTDA for the samples, the UFMG microscopy center for the images and the support of CNPQ, INCT Midas, CAPES and FAPEMIG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ballotin, F.C., Nascimento, M., Vieira, S.S. et al. Natural Mg silicates with different structures and morphologies: Reaction with K to produce K2MgSiO4 catalyst for biodiesel production. Int J Miner Metall Mater 27, 46–54 (2020). https://doi.org/10.1007/s12613-019-1891-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1891-9