Abstract
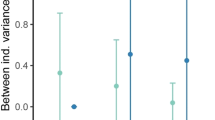
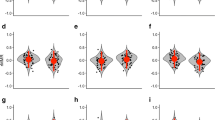
Phenotypic variation in behavior exists among species and populations, as well as among and within individuals. The pace-of-life syndrome hypothesis predicts covariation between life-history strategies, ranging from slow to fast, and behavior, ranging from shy, inactive, and flexible to bold, active, and less flexible. This covariation is expected to exist at multiple hierarchical levels, from the species down to the individual. We predict that fast-lived species will differ in average levels of behavior, and additionally show lower within-individual and among-individual variation than slow-lived ones. Shrews represent a highly suitable model to test these predictions, as they comprise a range of genera which differ tremendously in life-history strategy and metabolism. We performed repeated tests of boldness and aggression on 155 wild-caught individuals of five species of shrews, two species of the slow-lived genus Crocidura, two of the fast-lived genus Sorex, and one of the intermediate-paced genus Neomys. To compare not only average levels of behavior but also its variance components between those groups, we calculated coefficients of variation at within- and among-individual levels. Our results support our first prediction that, following the framework of pace-of-life-syndromes, fast-lived species should exhibit bolder behavior than slow-lived ones. However, our prediction of lower within- and among-individual variation in fast-lived species was not supported. Instead, our data suggest that other ecological factors might influence the expression of behavioral variation in shrew species, such as the variability in habitat choice and differences in anti-predator strategies.
Significance statement
The behavior and life history of animals are often structured into so-called pace-of-life syndromes (POLS), with slow-lived individuals being rather shy, inactive, and flexible and fast-lived individuals rather bold, active, and less flexible. Comparing the behavior in five species of shrews, we tested if such a gradient can also be found on the species level. While the average levels of species’ behavior indeed matched their pace of life, their individual behavior and behavioral stability did not. It was rather explained by an interplay of ecological and physiological factors, among them the variability in habitat choices and differences in anti-predator strategies. Our study shows that behavioral variation cannot be explained by just one factor like POLS at different hierarchical levels, but rather by a combination of factors including the animals’ life-history and ecological and physiological background.



Similar content being viewed by others
Data availability
The data set generated and analyzed during the current study is available from the corresponding author on reasonable request.
References
Archer J (1973) Tests for emotionality in rats and mice - review. Anim Behav 21:205–235
Baird HP, Patullo BW, Macmillan DL (2006) Reducing aggression between freshwater crayfish (Cherax destructor Clark: Decapoda, Parastacidae) by increasing habitat complexity. Aquac Res 37:1419–1428
Baxter-Gilbert J, Riley JL, Whiting MJ (2019) Bold new world: urbanization promotes an innate behavioral trait in a lizard. Behav Ecol Sociobiol 73:105
Bell AM, Sih A (2007) Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus). Ecol Lett 10:828–834
Benus RF, den Daas S, Koolhaas JM, van Oortmerssen GA (1990) Routine formation and flexibility in social and non-social behaviour of aggressive and non-aggressive male mice. Behaviour 112:176–193
Botero CA, Weissing FJ, Wright J, Rubenstein DR (2015) Evolutionary tipping points in the capacity to adapt to environmental change. P Natl Acad Sci USA 112:184–189
Bouskila A (1995) Interactions between predation risk and competition: a field study of kangaroo rats and snakes. Ecology 76:165–178
Brown C, Jones F, Braithwaite V (2005) In situ examination of boldness–shyness traits in the tropical poeciliid, Brachyraphis episcopi. Anim Behav 70:1003–1009
Careau V, Bininda-Emonds ORP, Thomas DW, Reale D, Humphries MM (2009) Exploration strategies map along fast-slow metabolic and life-history continua in muroid rodents. Funct Ecol 23:150–156
Christensen B (1996) Predator foraging capabilities and prey antipredator behaviours: pre-versus postcapture constraints on size-dependent predator-prey interactions. Oikos 76:368–380
Churchfield S (1990) The natural history of shrews. Cornell University Press, Ithaca
Churchfield S (1994) Foraging strategies of shrews, and the evidence from field studies. In: Merritt JF, Kirkland GL, Rose RK (eds) Advances in the biology of shrews., vol 18. Carnegie Museum of Natural History, Pittsburgh, USA, pp 77-87
Churchfield S, Rychlik L (2006) Diets and coexistence in Neomys and Sorex shrews in Białowieża forest, eastern Poland. J Zool 269:381–390
Cleasby IR, Nakagawa S, Schielzeth H (2015) Quantifying the predictability of behaviour: statistical approaches for the study of between-individual variation in the within-individual variance. Methods Ecol Evol 6:27–37
Coppens CM, de Boer SF, Koolhaas JM (2010) Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc B 365:4021–4028
Dahirel M, Vong A, Ansart A, Madec L (2017) Individual boldness is life stage-dependent and linked to dispersal in a hermaphrodite land snail. Ecol Res 32:751–755
Dall SRX, Bell AM, Bolnick DI, Ratnieks FL (2012) An evolutionary ecology of individual differences. Ecol Lett 15:1189–1198
Dammhahn M, Dingemanse NJ, Niemelä PT, Réale D (2018) Pace-of-life syndromes: a framework for the adaptive integration of behaviour, physiology and life history. Behav Ecol Sociobiol 72:62
Deventer SA, Uhl F, Bugnyar T, Miller R, Fitch WT, Schiestl M, Ringler M, Schwab C (2016) Behavioural type affects space use in a wild population of crows (Corvus corone). Ethology 122:881–891
Dingemanse NJ, Wright J, Kazem AJ, Thomas DK, Hickling R, Dawnay N (2007) Behavioural syndromes differ predictably between 12 populations of three-spined stickleback. J Anim Ecol 76:1128–1138
Dingemanse NJ, Barber I, Dochtermann NA (2020) Non-consumptive effects of predation: does perceived risk strengthen the genetic integration of behaviour and morphology in stickleback? Ecol Lett 23:107–118
Edwards PD, Palme R, Boonstra R (2016) Seasonal programming, not competition or testosterone, drives stress-axis changes in a partially-semelparous mammal. Horm Behav 85:96–101
Foster SA (1999) The geography of behaviour: an evolutionary perspective. Trends Ecol Evol 14:190–195
Frynta D, Kaftanová-Eliášová B, Žampachová B, Voráčková P, Sádlová J, Landová E (2018) Behavioural strategies of three wild-derived populations of the house mouse (Mus m. musculus and M. m. domesticus) in five standard tests of exploration and boldness: searching for differences attributable to subspecies and commensalism. Behav Process 157:133–141
Gracceva G, Herde A, Groothuis TGG, Koolhaas JM, Palme R, Eccard JA (2014) Turning shy on a winter’s day: effects of season on personality and stress response in Microtus arvalis. Ethology 120:753–767
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Holbrook CT, Wright CM, Pruitt JN (2014) Individual differences in personality and behavioural plasticity facilitate division of labour in social spider colonies. Anim Behav 97:177–183
Holtmann B, Lagisz M, Nakagawa S (2017) Metabolic rates, and not hormone levels, are a likely mediator of between-individual differences in behaviour: a meta-analysis. Funct Ecol 31:685–696
Houle D (1992) Comparing evolvability and variability of quantitative traits. Genetics 130:195–204
Innes DGL (1994) Life histories of the Soricidae. In: Merritt JF, Kirkland GL, Rose RK (eds) Advances in the biology of shrews, vol 18. Carnegie Museum of Natural History, Pittsburgh, pp 111–136
Jensen SP, Gray SJ, Hurst JL (2005) Excluding neighbours from territories: effects of habitat structure and resource distribution. Anim Behav 69:785–795
Knotts ER (2017) Influences of individual phenotypic traits on the habitat preferences of the sand fiddler crab, Uca pugilator. Behaviour 154:741–764
Kobler A, Maes GE, Humblet Y, Volckaert FA, Eens M (2011) Temperament traits and microhabitat use in bullhead, Cottus perifretum: fish associated with complex habitats are less aggressive. Behaviour 148:603–625
Koolhaas JM, Korte SM, de Boer SF, van der Vegt BJ, van Reenen CG, Hopster H, de Jong IC, Ruis MA, Blokhuis HJ (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav R 23:925–935
Kowalski K, Rychlik L (2018) The role of venom in the hunting and hoarding of prey differing in body size by the Eurasian water shrew, Neomys fodiens. J Mammal 99:351–362
Kowalski K, Marciniak P, Rosiński G, Rychlik L (2017) Evaluation of the physiological activity of venom from the Eurasian water shrew Neomys fodiens. Front Zool 14:46
Krushinska NL, Rychlik L (1994) Aggressiveness of a Neomys fodiens parous female towards conspecific and N. anomalus intruders. Acta Theriol 39:329–332
Lapiedra O, Chejanovski Z, Kolbe JJ (2017) Urbanization and biological invasion shape animal personalities. Glob Chang Biol 23:592–603
Laskowski KL, Pearish S, Bensky M, Bell AM (2015) Predictors of individual variation in movement in a natural population of threespine stickleback (Gasterosteus aculeatus). Adv Ecol Res 52:65–90
Mazza V, Eccard JA, Zaccaroni M, Jacob J, Dammhahn M (2018) The fast and the flexible: cognitive style drives individual variation in cognition in a small mammal. Anim Behav 137:119–132
Mettke-Hofmann C, Winkler H, Leisler B (2002) The significance of ecological factors for exploration and neophobia in parrots. Ethology 108:249–272
Michalak I (1983) Reproduction, maternal and social behaviour of the European water shrew under laboratory conditions. Acta Theriol 28:3–24
Michelangeli M, Goulet CT, Kang HS, Wong BB, Chapple DG (2018) Integrating thermal physiology within a syndrome: locomotion, personality and habitat selection in an ectotherm. Funct Ecol 32:970–981
Montiglio P-O, Dammhahn M, Messier GD, Réale D (2018) The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behav Ecol Sociobiol 72:116
Moraleva N (1989) Intraspecific interactions in the common shrew Sorex araneus in Central Siberia. Ann Zool Fenn 26:425–432
Moss S, Tittaferrante S, Way GP, Fuller A, Sullivan N, Ruhl N, McRobert SP (2015) Interactions between aggression, boldness and shoaling within a brood of convict cichlids (Amatitlania nigrofasciatus). Behav Process 121:63–69
Nagel A (1977) Torpor in the European white-toothed shrews. Experientia 33:1455–1456
Oliveira FG, Tapisso JT, Monarca RI, Cerveira AM, Mathias ML (2016) Phenotypic flexibility in the energetic strategy of the greater white-toothed shrew, Crocidura russula. J Therm Biol 56:10–17
Pearish S, Hostert L, Bell AM (2013) Behavioral type–environment correlations in the field: a study of three-spined stickleback. Behav Ecol Sociobiol 67:765–774
Promislow DE, Harvey PH (1990) Living fast and dying young: a comparative analysis of life-history variation among mammals. J Zool 220:417–437
Pucek Z (1981) Keys to vertebrates of Poland, mammals. PWN – Polish Scientific Publishers, Warszawa
Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Philos Trans R Soc B 365:4051–4063
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Romero LM (2002) Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24
Rychlik L (1998) Evolution of social systems in shrews. In: Wojcik JM, Wolsan M (eds) Evolution of shrews. Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland, pp 347–406
Rychlik L (2000) Habitat preferences of four sympatric species of shrews. Acta Theriol 45, Suppl. 1:173–190
Rychlik L, Zwolak R (2005) Behavioural mechanisms of conflict avoidance among shrews. Acta Theriol 50:289–308
Rychlik L, Zwolak R (2006) Interspecific aggression and behavioural dominance among four sympatric species of shrews. Can J Zool 84:434–448
Santicchia F, Gagnaison C, Bisi F, Martinoli A, Matthysen E, Bertolino S, Wauters LA (2018) Habitat-dependent effects of personality on survival and reproduction in red squirrels. Behav Ecol Sociobiol 72:134
Semlitsch RD, Gibbons JW (1988) Fish predation in size-structured populations of treefrog tadpoles. Oecologia 75:321–326
Sih A, Bell A, Johnson JC (2004a) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378
Sih A, Bell AM, Johnson JC, Ziemba RE (2004b) Behavioral syndromes: an integrative overview. Q Rev Biol 79:241–277
Sih A, Mathot KJ, Moirón M, Montiglio P-O, Wolf M, Dingemanse NJ (2015) Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol Evol 30:50–60
Sol D, Maspons J, Gonzalez-Voyer A, Morales-Castilla I, Garamszegi LZ, Møller AP (2018) Risk-taking behavior, urbanization and the pace of life in birds. Behav Ecol Sociobiol 72:59
Spiegel O, Leu ST, Sih A, Godfrey SS, Bull CM (2015) When the going gets tough: behavioural type-dependent space use in the sleepy lizard changes as the season dries. Proc R Soc B 282:20151768
Stamps JA (2007) Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol Lett 10:355–363
Stearns SC (1989) Trade-offs in life-history evolution. Funct Ecol 3:259–268
Strauss A, Mascher E, Palme R, Millesi E (2007) Sexually mature and immature yearling male European ground squirrels: a comparison of behavioral and physiological parameters. Horm Behav 52:646–652
Tapisso JT, Ramalhinho MG, Mathias ML, Rychlik L (2013) Ecological release: swimming and diving behavior of an allopatric population of the Mediterranean water shrew. J Mammal 94:29–39
Taylor L (1961) Aggregation, variance and the mean. Nature 189:732–735
Taylor JRE (1998) Evolution of energetic strategies in shrews. In: Wojcik JM, Wolsan M (eds) Evolution of shrews. Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland, pp 309–346
Verbeek ME, Drent PJ, Wiepkema PR (1994) Consistent individual differences in early exploratory behaviour of male great tits. Anim Behav 48:1113–1121
von Merten S, Zwolak R, Rychlik L (2017) Social personality: a more social shrew species exhibits stronger differences in personality types. Anim Behav 127:125–134
Westneat DF, Wright J, Dingemanse NJ (2015) The biology hidden inside residual within-individual phenotypic variation. Biol Rev 90:729–743
Wiersma P, Muñoz-Garcia A, Walker A, Williams JB (2007) Tropical birds have a slow pace of life. P Natl Acad Sci USA 104:9340–9345
Wilson DS (1998) Adaptive individual differences within single populations. Philos Trans R Soc B 353:199–205
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461
Wolf M, van Doorn GS, Leimar O, Weissing FJ (2007) Life-history trade-offs favour the evolution of animal personalities. Nature 447:581–585
Wright J, Bolstad GH, Araya-Ajoy YG, Dingemanse NJ (2019) Life-history evolution under fluctuating density-dependent selection and the adaptive alignment of pace-of-life syndromes. Biol Rev 94:230–247
Acknowledgments
We would like to thank Ana Cerveira, Diogo Barros, Fernando Madeira, Flávio Oliveira, Krzysztof Kowalski, and Pawel Kardynia for the help in the field; Anna Grozelier, Anna Kret, and Karla Bauer for the help in the field and with experiments; and Petri Niemelä for the advice in statistics. Further thanks are given to two anonymous reviewers for their helpful comments on an earlier version of our manuscript. Special thanks are given to Joaquim Tapisso for many fruitful discussions.
Funding
This work was supported by a Marie-Curie post-doc fellowship (PIEF-GA-2012-332,331) and an FCT post-doc fellowship (SFRH/BPD/118053/2016) to SvM; the budget of the Department of Systematic Zoology AMU; and CESAM (UID / AMB / 50,017/2019) through national funds by FCT/MCTES. NJD is supported by the German Science Foundation (grant no. DI 1694/1-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Capturing and testing were conducted with approval from the relevant governmental review board of each region: Regionalna Dyrekcja Ochrony Środowiska w Poznaniu (WPN-II.6401.143.2013.AG) for Poznań, Poland; Regierung von Oberbayern (55.1-8642-8-2007) for Starnberg, Germany; Regierungspräsidium Freiburg (35-9185.81/G-14/55) for Konstanz, Germany; and Ministério da Agricultura, do Mar do Ambiente e do Ordenamento do Território (01/2014/CAPT) for Portugal.
Additional information
Communicated by A. G. Ophir
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 530 kb)
Rights and permissions
About this article
Cite this article
von Merten, S., Dingemanse, N.J., Mathias, M.d.L. et al. Individual behavior, behavioral stability, and pace of life within and among five shrew species. Behav Ecol Sociobiol 74, 15 (2020). https://doi.org/10.1007/s00265-019-2793-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-019-2793-6