Abstract
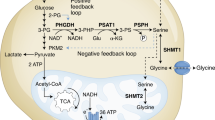
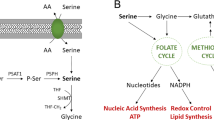
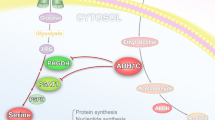
Serine racemase (SRR) catalyses not only the racemization but also the dehydration of l-serine and d-serine, resulting in the formation of pyruvate and ammonia. Although SRR activity is important in the central nervous system, SRR has not been linked to cancer metabolism before. Here we show that SRR supports proliferation of colorectal-cancer cells. We find that SRR expression is upregulated in colorectal adenoma and adenocarcinoma lesions compared with non-neoplastic mucosa in human colorectal-cancer specimens. SRR-mediated dehydration of serine contributes to the pyruvate pool in colon-cancer cells, enhances proliferation, maintains mitochondrial mass and increases basal reactive oxygen species production, which has anti-apoptotic effects. Moreover, SRR promotes acetylation of histone H3 by maintaining intracellular acetyl-CoA levels. Inhibition of SRR suppresses growth of colorectal tumours in mice and augments the efficacy of 5-fluorouracil treatment. Our findings highlight a previously unknown mechanism through which a racemase supports cancer-cell growth and suggest that SRR might be a molecular target for colorectal-cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. RNA-seq data that support the findings of this study have been deposited in GEO with the accession code GSE128804. The previously published data of colorectal-cancer cells that have been reanalysed here are available under ArrayExpress accession code E-MTAB-2971 (ref. 21). Data in Fig. 1a are publicly available online at www.oncomine.org. Source data for Figs. 2, 3 and 5 and Extended Data Fig. 2 are available online.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J. Clin. 68, 394–424 (2018).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Vyas, S., Zaganjor, E. & Haigis, M. C. Mitochondria and cancer. Cell 166, 555–566 (2016).
Porporato, P. E., Filigheddu, N., Pedro, J. M. B., Kroemer, G. & Galluzzi, L. Mitochondrial metabolism and cancer. Cell Res. 28, 265–280 (2018).
Chandel, N. S. Evolution of mitochondria as signaling organelles. Cell Metab. 22, 204–206 (2015).
Martinez-Reyes, I. et al. TCA cycle and mitochondrial membrane potential are necessary for diverse biological functions. Mol. Cell 61, 199–209 (2016).
Wang, C. & Youle, R. J. The role of mitochondria in apoptosis. Annul. Rev. Genet. 43, 95–118 (2009).
Cruz, M. D. et al. Metabolic reprogramming of the premalignant colonic mucosa is an early event in carcinogenesis. Oncotarget 8, 20543–20557 (2017).
Wen, Y. A. et al. The mitochondrial retrograde signaling regulates Wnt signaling to promote tumorigenesis in colon cancer. Cell Death Differ. https://doi.org/10.1038/s41418-018-0265-6 (2019).
Satoh, K. et al. Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proc. Natl Acad. Sci. USA 114, E7697–E7706 (2017).
Wolosker, H., Blackshaw, S. & Snyder, S. H. Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc. Natl Acad. Sci. USA 96, 13409–13414 (1999).
De Miranda, J., Panizzutti, R., Foltyn, V. N. & Wolosker, H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-d-aspartate (NMDA) receptor coagonist d-serine. Proc. Natl Acad. Sci. USA 99, 14542–14547 (2002).
Foltyn, V. N. et al. Serine racemase modulates intracellular d-serine levels through an alpha,beta-elimination activity. J. Biol. Chem. 280, 1754–1763 (2005).
Mothet, J. P. et al. d-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc. Natl Acad. Sci. USA 97, 4926–4931 (2000).
Kim, P. M. et al. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc. Natl Acad. Sci. USA 102, 2105–2110 (2005).
Campanini, B., Spyrakis, F., Peracchi, A. & Mozzarelli, A. Serine racemase: a key player in neuron activity and in neuropathologies. Front. Biosci. 18, 1112–1128 (2013).
Raboni, S. et al. The energy landscape of human serine racemase. Front. Mol. Biosci. 5, https://doi.org/10.3389/fmolb.2018.00112 (2019).
Marchetti, M. et al. ATP binding to human serine racemase is cooperative and modulated by glycine. FEBS J. 280, 5853–5863 (2013).
Bazzocco, S. et al. Highly expressed genes in rapidly proliferating tumor cells as new targets for colorectal cancer treatment. Clin. Cancer Res. 21, 3695–3704 (2015).
Lozoya, O. A. et al. Mitochondrial acetyl-CoA reversibly regulates locus-specific histone acetylation and gene expression. Life Sci. Alliance 2, https://doi.org/10.26508/lsa.201800228 (2019).
Schieber, M. & Chandel, N. S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Lee, S. R. et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277, 20336–20342 (2002).
Pommier, Y., Sordet, O., Antony, S., Hayward, R. L. & Kohn, K. W. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene 23, 2934–2949 (2004).
Nita, M. E. et al. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl-2 family proteins. Br. J. Cancer 78, 986–992 (1998).
Sasabe, J. et al. d-serine is a key determinant of glutamate toxicity in amyotrophic lateral sclerosis. EMBO J. 26, 4149–4159 (2007).
Hagiwara, H., Iyo, M. & Hashimoto, K. Neonatal disruption of serine racemase causes schizophrenia-like behavioral abnormalities in adulthood: clinical rescue by d-serine. PLoS One 8, e62438 (2013).
Watanabe, A. et al. Serine racemase inhibition induces nitric oxide-mediated neurovascular protection during cerebral ischemia. Neuroscience 339, 139–149 (2016).
Beltran-Castillo, S. et al. d-serine released by astrocytes in brainstem regulates breathing response to CO2 levels. Nature Commun 8, 838 (2017).
Snell, K., Natsumeda, Y., Eble, J. N., Glover, J. L. & Weber, G. Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma. Br. J. Cancer 57, 87–90 (1988).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Cai, L., Sutter, B. M., Li, B. & Tu, B. P. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol. Cell 42, 426–437 (2011).
Karczmarski, J. et al. Histone H3 lysine 27 acetylation is altered in colon cancer. Clin. Proteom 11, 24 (2014).
Kroll, W., Loffler, M. & Schneider, F. Energy parameters, macromolecular synthesis and cell cycle progression of in vitro grown Ehrlich ascites tumor cells after inhibition of oxidative ATP synthesis by oligomycin. Zeitschrift für Naturforschung. C 38, 604–612 (1983).
Loffer, M. & Schneider, F. Further characterization of the growth inhibitory effect of rotenone on in vitro cultured Ehrlich ascites tumour cells. Mol. Cell. Biochem. 48, 77–90 (1982).
Molina, J. R. et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 24, 1036–1046 (2018).
Kaldma, A. et al. An in situ study of bioenergetic properties of human colorectal cancer: the regulation of mitochondrial respiration and distribution of flux control among the components of ATP synthasome. Int. J. Biochem. Cell Biol. 55, 171–186 (2014).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Sellers, K. et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J. Clin. Invest. 125, 687–698 (2015).
Boyle, K. A. et al. Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J. Biol. Chem. 293, 14891–14904 (2018).
Spandidos, A., Wang, X., Wang, H. & Seed, B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 38, D792–D799 (2010).
Konya, Y., Taniguchi, M. & Fukusaki, E. Novel high-throughput and widely-targeted liquid chromatographyetime of flight mass spectrometry method for d-amino acids in foods. J. Biosci. Bioeng. 123, 126–133 (2017).
Acknowledgements
We thank M. Kohara, E. Maeno and T. Sawamura from Department of Pathology, Osaka University Graduate School of Medicine, and H. Omori and K. Murata from Core Instrumentation Facility, Research Institute for Microbial Diseases, Osaka University, for their technical assistance. This study was supported by E. Oiki and the Center for Medical Research and Education, Osaka University Graduate School of Medicine. This work was supported in part by JSPS KAKENHI grant number T16K086490, T17K195550, T18K151220, T18K150780, T18K150790, T18K070680, A19H034520, T19K165570 and T19K165850.
Author information
Authors and Affiliations
Contributions
K.O. carried out most of the experiments, analysed data and managed clinical information. D.O. performed RNA-seq analysis and analysed data. M.T, Y.U. and E.F. performed stable-isotope tracing, LC–MS and d-amino acids analysis. S.N., S.T., M.K., K.K., Y.H., N.W., and J.-i.I. contributed to preparation of materials and provided advice on project planning and data interpretation. K.O. and E.M. designed the project, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Christoph Schmitt.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SRR protein expression levels in colorectal carcinoma cases.
a. Representative images of two independent experiments of immunohistochemistry of SRR in colorectal carcinoma tissues with high (score 2) and low (score 1) signal intensity, which were used to calculate H-scores. Scale bars, 200 μm. b. SRR protein expression levels in 83 colorectal carcinoma cases according to tumor progression (T grade), the degree of lymph node metastasis (N grade), or the existence of other organ metastasis (M grade) were evaluated by calculating H-scores. The number of cases were T1 (n = 12), T2 (n = 13), T3 (n = 47), T4 (n = 11), N0 (n = 45), N1/2/3 (n = 38), M0 (n = 66) and M1 (n = 17). The middle line indicates the mean, and the top and bottom line indicate standard deviation. P-values were determined by two-tailed Mann–Whitney U-test.
Extended Data Fig. 2 Histone acetylation and cell proliferation capability induced by SRR knockout and rescued by pyruvate or acetate supplementation.
a. Relationships between the cell number and the relative SRR mRNA level obtained from Fig. 2b, d. b. pyruvate concentration in DMEM without pyruvate with 10% FBS used in all the experiments unless otherwise mentioned. Data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. c. The levels of M + 3-labeled D-serine in EV and SRR-KO HCT116 cells cultured with 13C3-L-serine for 6 hours. Data are means ± SD of n = 3 biologically independent samples. d. Intracellular lactate levels in EV and SRR-KO DLD-1 and HCT116 cells. Data are means ± SD of n = 4 biologically independent samples and are representative of two independent experiments. e. Immunoblotting of SRR in DLD-1 EV and SRR-KO cells transfected with control empty vector (pVec), Wild-type (WT)-SRR and Q155D-SRR. Blots are representative of n = 3 biologically independent experiments. f. Relative number of cells at 5 days after seeding of 2 × 104 cells. Data are means ± SD of n = 3 biologically independent samples and are representative of three independent experiments. g. Relative number of EV and SRR-KO DLD-1 and HCT116 cells cultured in medium containing 5 mM acetate or vehicle (DDW) for 6 days. Data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. h. Immunoblotting of acetylated histone H3, acetylated histone H3K27, and histone H3 in DLD-1 EV and SRR-KO cells treated with 1 mM pyruvate or vehicle (DDW) for 72 hours. Blots are representative of n = 2 biologically independent experiments. i. Immunoblotting of acetylated histone H3 and histone H3 in DLD-1 EV and SRR-KO cells transfected with control empty vector (pVec), Wild-type (WT)-SRR and Q155D-SRR. Blots are representative of n = 2 biologically independent experiments. j. Representative images of two independent experiments of immunohistochemistry of acetyl-histone H3 in xenograft tumors of DLD-1 EV and SRR-KO cells with high (score 3), intermediate (score 2) and low (score 1) signal intensity, which were used to calculate H-scores. Scale bars, 100 μm. P-values were determined by two-tailed Student’s t-test.
Extended Data Fig. 3 The mitochondrial mass, mitochondrial morphology and oxygen consumption rate of SRR-KO cells were rescued by pyruvate or acetate supplementation.
a. Transmission electron microscopy images showing the morphology of mitochondria in WiDr cells stably expressing SRR-GFP or GFP. Blue and orange dotted lines indicate mitochondria. Scale bars represent 2 and 0.5 µm (magnified images). Images are representative of n = 2 biologically independent experiments. b,c. Quantification of mitochondrial elongation (b) and number of cristae folds per micrometer of mitochondria (c) in WiDr cells stably expressing SRR-GFP or GFP using ImageJ. Mitochondrial elongation was derived from the reciprocal of circularity. Data are means ± SD of n = 59 (GFP) and 94 (SRR-GFP) mitochondria. d-f. Immunofluorescence assay (d) and Flow cytometry analysis (e) using MitoTracker Deep Red and oxygen consumption rate (f) of EV and SRR-KO HCT116 cells after incubation with 1 mM pyruvate, 5 mM acetate or vehicle (DDW) for 72 hours. Images are representative of n = 3 biologically independent experiments (d), and data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments (e and f). g. Transmission electron microscopy images showing the morphology of mitochondria in EV and SRR-KO DLD-1 cells after incubation with 1 mM pyruvate, 5 mM acetate or vehicle (DDW) for 72 hours. Blue and orange dotted lines indicate mitochondria. Scale bars represent 2 and 0.5 µm (magnified images). Images are representative of n = 2 biologically independent experiments. h,i. Quantification of mitochondrial elongation (h) and number of cristae folds per micrometer of mitochondria (i) in EV and SRR-KO DLD-1 cells after incubation with 1 mM pyruvate, 5 mM acetate or vehicle (DDW) for 72 hours using ImageJ. Mitochondrial elongation was derived from the reciprocal of circularity. Data are means ± SD of n = 104 (EV vehicle), 92 (sgSRR_2 vehicle), 113 (sgSRR_2 pyruvate) and 95 (sgSRR_2 acetate) mitochondria. j. Flow cytometry analysis using MitoTracker Deep Red of DLD-1 EV and SRR-KO cells transfected with control empty vector (pVec), Wild-type (WT)-SRR and Q155D-SRR. Data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. P-values were determined by two-tailed Student’s t-test.
Extended Data Fig. 4 Lipid content in SRR-KO cells rescued by pyruvate or acetate supplementation.
a. Lipid droplet content assessed by Nile red staining in EV and SRR-KO DLD-1 and HCT116 cells after incubation with 1 mM pyruvate, 5 mM acetate or vehicle (DDW) for 72 hours, and WiDr cells stably expressing SRR-GFP or GFP. Nuclei (blue), lipid droplet (red). Images are representative of n = 3 biologically independent experiments.
Extended Data Fig. 5 The effect of pyruvate or acetate supplementation or NAC treatment on cellular ROS levels.
a. Flow cytometry analysis using CellROX Deep Red of EV and SRR-KO DLD-1 and HCT116 cells after incubation with 1 mM pyruvate, 5 mM acetate or vehicle (DDW) for 72 hours. Data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. b. Flow cytometry analysis using CellROX Deep Red of EV and SRR-KO DLD-1 and HCT116 cells, and WiDr cells stably expressing SRR-GFP or GFP after incubation with 1 mM N-acetyl-L-cysteine (NAC) for 24 hours. Data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. c. The proportion of the dead cells after incubation with 1 mM NAC for 5 days. Data are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. P-values were determined by two-tailed Student’s t-test.
Extended Data Fig. 6 The effect of pyruvate or acetate supplementation on apoptosis of SRR-KO cells.
a. Immunofluorescence analysis of cleaved caspase 3 in EV and SRR-KO DLD-1 and HCT116 cells after incubation with 1 mM pyruvate, 5 mM acetate or vehicle (DDW) for 72 hours and subsequent treatment with 0.5 µM staurosporine for 4 hours. Data are means ± SD of n = 4 biologically independent samples; 200–500 cells per sample were analyzed. Data are representative of three independent experiments. b. Number of cells of WiDr cells stably expressing SRR-GFP or GFP after treatment with 5-FU for 5 days. Data are ratios relative to number of cells without 5-FU, and are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. c. Number of cells of DLD-1 EV and SRR-KO cells transfected with control empty vector (pVec), Wild-type (WT)-SRR and Q155D-SRR after treatment with 5-FU for 5 days. Data are ratios relative to number of cells without 5-FU, and are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. d-f. EV and SRR-KO HCT116 cells (1 × 106) were subcutaneously injected into 6-week-old female nude mice, which were treated with 40 mg/kg 5-FU or normal saline (NS) every second or third day after the major axis of the tumor reached 1 mm. Tumor size was recorded every second or third day (d) and tumors were weighed after harvesting at the end of the experiment (e). Ratios of weights of the tumors treated with 5-FU to those of the tumors treated with NS in each group are shown in (f). Data are means ± SD of n = 5 tumors per group. P-values were determined by two-tailed Student’s t-test in a-c, and by two-tailed Mann–Whitney U-test in d-f.
Extended Data Fig. 7 Weight and organ morphology of mice treated with Phenazine methosulphate (PMS) or normal saline (NS) and the effect of combination of PMS and 5-FU or CDDP.
a. Weight of mice transplanted with EV or SRR-KO cells of DLD-1 and treated with PMS or normal saline (NS). Data are presented as mean ± SD of n = 6 mice per group. b. Hematoxylin and eosin (HE) stains of liver, kidney, lung and myocardium of mice transplanted with EV or SRR-KO cells of DLD-1 and treated with PMS or NS. Scale bars, 500 μm in figures of liver, lung and myocardium, and 200 µm in figures of kidney. Images are representative of six mice per group c. Number of cells of EV and SRR-KO DLD-1 and HCT116 cells after incubation with vehicle (DDW), phenazine methosulphate (PMS), 5-fluorouracil (5-FU) or cisplatin (CDDP), and PMS with 5-FU or CDDP for 5 days. Data are ratios relative to number of cells treated with vehicle, and are means ± SD of n = 3 biologically independent samples and are representative of two independent experiments. P-values were determined by two-tailed Student’s t-test.
Extended Data Fig. 8 The mRNA expression levels of genes related glycolysis and serine dehydratase.
a. Representative glycolytic pathway. b. Heat map of RNA-seq data related to glycolytic pathway in EV and SRR-KO cells of DLD-1 and HCT116 cells. c. FPKM of serine racemase and serine dehydratase in EV cells of DLD-1 and HCT116 cells, derived from RNA-seq data. d. Serine dehydratase mRNA expression in non-neoplastic and tumor of colorectal tissue from the ONCOMINE datasets. n = 22 non-neoplastic mucosa and n = 183 adenocarcinoma in the TCGA dataset, and n = 10 non-neoplastic mucosa and n = 5 adenoma in Skrzypczak’s dataset. P-values were determined by two-tailed Mann–Whitney U-test.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Tables 1 and 2
Source data
Source Data Fig. 2
Unprocessed western blots in Fig. 2
Source Data Fig. 3
Unprocessed western blots in Fig. 3
Source Data Fig. 5
Unprocessed western blots in Fig. 5
Source Data Extended Data Fig. 2
Unprocessed western blots in Extended Data Fig. 2
Rights and permissions
About this article
Cite this article
Ohshima, K., Nojima, S., Tahara, S. et al. Serine racemase enhances growth of colorectal cancer by producing pyruvate from serine. Nat Metab 2, 81–96 (2020). https://doi.org/10.1038/s42255-019-0156-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-019-0156-2
This article is cited by
-
Detection and analysis of chiral molecules as disease biomarkers
Nature Reviews Chemistry (2023)
-
Melatonin inhibits bladder tumorigenesis by suppressing PPARγ/ENO1-mediated glycolysis
Cell Death & Disease (2023)
-
Macrophages rely on extracellular serine to suppress aberrant cytokine production
Scientific Reports (2021)
-
Mitochondrial matrix protein C14orf159 attenuates colorectal cancer metastasis by suppressing Wnt/β-catenin signalling
British Journal of Cancer (2021)