Abstract
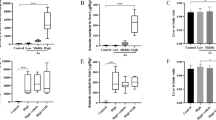
Arsenic is a potent and toxic heavy metal found in the environment that causes health problems, including liver disease, in humans and animals. Chlorogenic acid (CA) is the most abundant caffeoylquinic acid isomer present in plants. This study aims to assess how CA protects the liver tissue following sodium arsenite (NaAsO2)-induced toxicity in mice. Male Swiss mice were allocated into 5 groups: Control, intragastrically administered CA (200 mg/kg), intragastrically administered NaAsO2 (5 mg/kg), and two groups administered with CA (100 and 200 mg/kg) and NaAsO2. CA was administered 30 min before NaAsO2 and all the mice were treated daily for 28 days. To investigate the biochemical, histopathological, immunohistochemical, and molecular changes, blood and liver samples were collected. NaAsO2 treatment increased the liver function biomarkers such as alanine transaminase, aspartate transaminase, alkaline phosphatase, and total bilirubin. Lipid and nitric oxide production was elevated. Glutathione content and the activities of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase decreased, indicating a disturbance in redox homeostasis. Histopathological examination revealed a granular degeneration of hepatocytes, infiltration of inflammatory cells, and centrilobular hepatocyte necrosis. Furthermore, tumor necrosis factor-α and interleukin-1β were upregulated upon NaAsO2 treatment, suggesting the induction of inflammation. Moreover, NaAsO2 triggered apoptosis in the liver by upregulating Bax and caspase-3 and downregulating Bcl-2. However, CA abrogated the biochemical, molecular, and histological changes, reflecting its hepatoprotective role in response to NaAsO2 treatment. Our findings demonstrate that CA could be a potential therapeutic to minimize NaAsO2-induced hepatic injury.









Similar content being viewed by others
References
Jalaludeen MA, Ha TW, Lee R, Kim HJ, Do TJ, Park C, Heo TY, Lee YW, Song H (2016) Biochanin A ameliorates arsenic-induced hepato- and hematotoxicity in rats. Molecules.https://doi.org/10.3390/molecules21010069
Kulik-Kupka K, Koszowska A, Brończyk-Puzoń A, Nowak J, Gwizdek K, Zubelewicz-Szkodzińska B (2016) Arsenic–Poison or medicine? Med Pr 67(1):89–96. https://doi.org/10.13075/mp.5893.00322
Thangapandiyan S, Ramesh M, Hema T, Miltonprabu S, Uddin MS, Nandhini V, Bavithra Jothi G (2019) Sulforaphane potentially ameliorates arsenic induced hepatotoxicity in albino wistar rats: implication of PI3K/Akt/Nrf2 signaling pathway. Cell Physiol Biochem 52(5):1203–1222. https://doi.org/10.33594/000000082
Azizur Rahman M, Hasegawa H, Mahfuzur Rahman M, MazID Miah MA, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicol Environ Saf 69(2):317–324 doi:S0147-6513(07)00003-6
Pace C, Dagda R, Angermann J (2017) AntioxIDants protect against arsenic induced mitochondrial cardio-toxicity. Toxics.https://doi.org/10.3390/toxics5040038
Flora SJ, Mehta A, Gupta R (2009) Prevention of arsenic-induced hepatic apoptosis by concomitant administration of garlic extracts in mice. Chem Biol Interact 177(3):227–233. doi:S0009-2797(08)00479-1
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. doi:https://doi.org/10.1007/978-3-7643-8340-4_6
Ling S, Shan Q, Liu P, Feng T, Zhang X, Xiang P, Chen K, Xie H, Song P, Zhou L, Liu J, Zheng S, Xu X (2017) Metformin ameliorates arsenic trioxide hepatotoxicity via inhibiting mitochondrial complex I. Cell Death Dis 8(11):e3159–e3159. doi:https://doi.org/10.1038/cddis.2017.482
Al-Brakati AY, Kassab RB, Lokman MS, Elmahallawy EK, Amin HK, Abdel Moneim AE (2019) Role of thymoquinone and ebselen in the prevention of sodium arsenite-induced nephrotoxicity in female rats. Hum Exp Toxicol 38(4):482–493. doi:https://doi.org/10.1177/0960327118818246
Li S, Wei BK, Wang J, Dong G, Wang X (2019) Taurine supplementation ameliorates arsenic-induced hepatotoxicity and oxidative stress in mouse. In: Hu J, Piao F, Schaffer SW, El Idrissi A, Wu J-Y (eds). Taurine 11. Springer, Singapore, pp 463–470
Singh AP, Goel RK, Kaur T (2011) Mechanisms pertaining to arsenic toxicity. Toxicol Int 18(2):87–93. doi:https://doi.org/10.4103/0971-6580.84258
Fouad AA, Al-Mulhim AS, Jresat I (2012) Telmisartan treatment attenuates arsenic-induced hepatotoxicity in mice. Toxicology 300(3):149–157. doi:https://doi.org/10.1016/j.tox.2012.06.015
Wu R, Wu X, Wang H, Fang X, Li Y, Gao L, Sun G, Pi J, Xu Y (2017) Strain differences in arsenic-induced oxidative lesion via arsenic biomethylation between C57BL/6J and 129 × 1/SvJ mice. Sci Rep 7:44424. doi:https://doi.org/10.1038/srep44424
Farah A, de Paula Lima J (2019) Consumption of chlorogenic AcIDs through coffee and health implications. Beverages.https://doi.org/10.3390/beverages5010011
Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, ShumzaID M, Ahmad F, Babazadeh D, FangFang X, Modarresi-Ghazani F, WenHua L, XiaoHui Z (2018) Chlorogenic acID (CGA): a pharmacological review and call for further research. Biomed Pharmacother 97:67–74. https://doi.org/10.1016/j.biopha.2017.10.064
Kataria R, Khatkar A (2019) In-silico design, synthesis, ADMET studies and biological evaluation of novel derivatives of chlorogenic acID against urease protein and H. Pylori bacterium BMC Chem 13(1):41. https://doi.org/10.1186/s13065-019-0556-0
Wang X, Xi Y, Zeng X, Zhao H, Cao J, Jiang W (2018) Effects of chlorogenic acID against aluminium neurotoxicity in ICR mice through chelation and antioxIDant actions. J Funct Foods 40:365–376. https://doi.org/10.1016/j.jff.2017.11.013
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63
Shephard MD, Peake MJ (1986) Quantitative method for determining serum alkaline phosphatase isoenzyme activity I. Guanidine hydrochloride: new reagent for selectively inhibiting major serum isoenzymes of alkaline phosphatase. J Clin Pathol 39(9):1025–1030
Schmidt M, Eisenburg J (1975) [Serum bilirubin determination in newborn infants. A new micromethod for the determination of serum of plasma bilirubin in newborn infants]. Fortschr Med 93(30):1461–1466
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluIDs. Anal Biochem 126(1):131–138
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34(3):497–500
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70(1):158–169. doi:0022-2143(67)90076-5
Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS (1998) Disruption of redox homeostasis in the transforming growth factor-alpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J Biol Chem 273(25):15846–15853
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Dkhil MA, al-Quraishy S, Abdel Moneim AE (2018) Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via antioxidant and anti-inflammatory effects. Inflammopharmacology. doi:https://doi.org/10.1007/s10787-017-0439-8
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. doi:clinchem.2008.112797
Chen Z, Yang Y, Mi S, Fan Q, Sun X, Deng B, Wu G, Li Y, Zhou Q, Ruan Z (2019) Hepatoprotective effect of chlorogenic acID against chronic liver injury in inflammatory rats. J Funct Foods 62:103540. https://doi.org/10.1016/j.jff.2019.103540
Kim H, Pan JH, Kim SH, Lee JH, Park JW (2018) Chlorogenic acid ameliorates alcohol-induced liver injuries through scavenging reactive oxygen species. Biochimie 150:131–138. doi:S0300-9084(18)30131-7
Ali N, RashID S, Nafees S, Hasan SK, ShahID A, Majed F, Sultana S (2017) Protective effect of Chlorogenic acID against methotrexate induced oxIDative stress, inflammation and apoptosis in rat liver: an experimental approach. Chem Biol Interact 272:80–91 doi:S0009-2797(17)30140-0
Chen C, Jiang X, Hu Y, Zhang Z (2013) The protective role of resveratrol in the sodium arsenite-induced oxidative damage via modulation of intracellular GSH homeostasis. Biol Trace Elem Res 155(1):119–131. doi:https://doi.org/10.1007/s12011-013-9757-x
Hosseinzadeh A, Houshmand G, Goudarzi M, Sezavar SH, Mehrzadi S, Mansouri E, Kalantar M (2019) Ameliorative effect of gallic acid on sodium arsenite-induced spleno-, cardio- and hemato-toxicity in rats. Life Sci 217:91–100. doi:S0024-3205(18)30771-9
Mehrzadi S, Fatemi I, Malayeri AR, Khodadadi A, Mohammadi F, Mansouri E, Rashno M, Goudarzi M (2018) Ellagic acID mitigates sodium arsenite-induced renal and hepatic toxicity in male Wistar rats. Pharmacol Rep 70(4):712–719
Hamoud AR, Weaver L, Stec DE, Hinds TD Jr (2018) Bilirubin in the liver-gut signaling axis. Trends Endocrinol Metab 29(3):140–150
Narita M, Shimizu S, Ito T, Chittenden T, Lutz RJ, Matsuda H, Tsujimoto Y (1998) Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc Natl Acad Sci USA 95(25):14681–14686. doi:https://doi.org/10.1073/pnas.95.25.14681
Shi A, Shi H, Wang Y, Liu X, Cheng Y, Li H, Zhao H, Wang S, Dong L (2018) Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acID on acute liver injury. Int Immunopharmacol 54:125–130
Acknowledgement:
This study was supported by Research Supporting Project (RSP-2019/23), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
All the authors contributed equally to this work.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dkhil, M.A., Abdel Moneim, A.E., Bauomy, A.A. et al. Chlorogenic acid prevents hepatotoxicity in arsenic-treated mice: role of oxidative stress and apoptosis. Mol Biol Rep 47, 1161–1171 (2020). https://doi.org/10.1007/s11033-019-05217-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05217-4