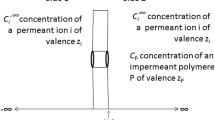
Abstract
We have developed a model to study the kinetics of the redistribution of ions and molecules through a semipermeable membrane in complex mixtures of substances penetrating and nonpenetrating through a membrane. It takes into account the degree of dissociation of these substances, their initial concentrations in solutions separated by a membrane, and volumes of these solutions. The model is based on the assumption that only uncharged particles (molecules or ion pairs) diffuse through a membrane (and not ions as in the Donnan model). The developed model makes it possible to calculate the temporal dependencies of concentrations for all processing ions and molecules at system transition from the initial state to equilibrium. Under equilibrium conditions, the ratio of ion concentrations in solutions separated by a membrane obeys the Donnan distribution. The Donnan effect is the result of three factors: equality of equilibrium concentrations of penetrating molecules on each side of a membrane, dissociation of molecules into ions, and Le Chatelier’s principle. It is shown that the Donnan distribution (irregularity of ion distribution) and accordingly absolute value of the Donnan membrane potential increases if: (i) the nonpenetrating salt concentration (in one of the solutions) and its dissociation constant increases, (ii) the total penetrating salt concentration and its dissociation constant decreases, and (iii) the volumes ratio increases (between solutions with and without a nonpenetrating substance). It is shown also that only a slight difference between the degrees of dissociation of two substances can be used for their membrane separation.

Similar content being viewed by others
References
Al-Obaidi MA, Kara-Zaitri C, Mujtaba IM (2017) Scope and limitations of the irreversible thermodynamics and the solution diffusion models for the separation of binary and multi-component systems in reverse osmosis process. Comput Chem Eng 100:48–79. https://doi.org/10.1016/j.compchemeng.2017.02.001
Cohen H, Cooley JW (1965) The numerical solution of the time-dependent Nernst-Planck equations. Biophys J 5:145–162. https://doi.org/10.1016/S0006-3495(65)86707-8
Davis TA (2000) Donnan dialysis. In: Wilson ID, Adlard ER, Cooke M, Poole CF (eds) Encyclopedia of separation science. Academic Press, London, pp 1701–1707
Déon S, Escoda A, Fievet P, Salut R (2013) Prediction of single salt rejection by NF membranes: an experimental methodology to assess physical parameters from membrane and streaming potentials. Desalination 315:37–45. https://doi.org/10.1016/j.desal.2012.09.005
Donnan FG (1924) The theory of membrane equilibria. Chem Rev 1:73–90. https://doi.org/10.1021/cr60001a003
Donnan FG (1995) Theory of membrane equilibria and membrane potentials in the presence of non-dialysing electrolytes. A contribution to physical-chemical physiology. J Membr Sci 100:45–55. https://doi.org/10.1016/0376-7388(94)00297-C
Duffey ME, Fennell Evans D, Cussler EL (1978) Simultaneous diffusion of ions and ion pairs across liquid membranes. J Membr Sci 3:1–14. https://doi.org/10.1016/S0376-7388(00)80407-X
Fridman-Bishop N, Tankus KA, Freger V (2018) Permeation mechanism and interplay between ions in nanofiltration. J Membr Sci 548:449–458. https://doi.org/10.1016/j.memsci.2017.11.050
Galach M, Waniewski J (2012) Membrane transport of several ions during peritoneal dialysis: mathematical modeling. Artif Organs 36:E163–E178. https://doi.org/10.1111/j.1525-1594.2012.01484.x
Galama AH, Post JW, Hamelers HVM, Nikonenko VV, Biesheuvel PM (2016) On the origin of the membrane potential arising across densely charged ion exchange membranes: how well does the Teorell-Meyer-Sievers theory work? J Membr Sci Res 2:128–140. https://doi.org/10.22079/jmsr.2016.20311
Gimmi T, Alt-Epping P (2018) Simulating Donnan equilibria based on the Nernst-Planck equation. Geochim Cosmochim Acta 232:1–13. https://doi.org/10.1016/j.gca.2018.04.003
Grzegorczyn S, Ślęzak A (2006) Time characteristics of electromotive force in single-membrane cell for stable and unstable conditions of reconstructing of concentration boundary layers. J Membr Sci 280:485–493. https://doi.org/10.1016/j.memsci.2006.02.004
Higa M, Kira A (1992) Theory and simulation of ion transport in nonstationary states against concentration gradients across ion-exchange membranes. J Phys Chem 96:9518–9523. https://doi.org/10.1021/j100202a081
Johnson KS, Pytkowicz RM (1978) Ion association of Cl− with H+, Na+, K+, Ca2+, and Mg2+ in aqueous solutions at 25° C. Am J Sci 278:1428–1447. https://doi.org/10.2475/ajs.278.10.1428
Kim DY, Lee MH, Boram G, Kim JH, Lee S, Yang DR (2010) Modeling of solute transport in multi-component solution for reverse osmosis membranes. Desalination Water Treat 15:20–28. https://doi.org/10.5004/dwt.2010.1662
Kondepudi D, Prigogine I (1998) Modern thermodynamics. From heat engines to dissipative structures. John Wiley & Sons, New York
Kosterin SA, Cherny AP (1991) Gibbs-Donnan equilibrium in the system membrane vesicules – incubation medium. Biofizika 36:826–829. (In Russian)
Kozmai A, Chérif M, Dammak L, Bdiri M, Larchet C, Nikonenko V (2017) Modelling non-stationary ion transfer in neutralization dialysis. J Membr Sci 540:60–70. https://doi.org/10.1016/j.memsci.2017.06.039
Kumaran M, Bajpai S (2015) Application of extended Nernst Planck model in nano filtration process –a critical review. Int J Eng Res Rev 3:40–49
Kurbel S (2011) Donnan effect on chloride ion distribution as a determinant of body fluid composition that allows action potentials to spread via fast sodium channels. Theor Biol Med Model 8:16. https://doi.org/10.1186/1742-4682-8-16
Lang GE, Stewart PS, Vella D, Waters SL, Goriely A (2014) Is the Donnan effect sufficient to explain swelling in brain tissue slices? J Roy Soc Interface 11:20140123. https://doi.org/10.1098/rsif.2014.0123
Luo J, Wu C, Wu Y, Xu T (2013) Diffusion dialysis of hydrochloric acid with their salts: effect of co-existence metal ions. Sep Purif Technol 118:716–722. https://doi.org/10.1016/j.seppur.2013.08.014
Marcus Y, Hefter G (2006) Ion pairing. Chem Rev 106:4585–4621. https://doi.org/10.1021/cr040087x
Mazur I, Kosterin S, Veklich T, Shkrabak O (2014) Gibbs-Donnan potential as a tool for membrane vesicles polarization. J Biophys Chem 5:78–89. https://doi.org/10.4236/jbpc.2014.52009
Moshtarikhah S, Oppers NAW, de Groot MT, Keurentjes JTF, Schouten JC, van der Schaaf J (2017) Nernst-Planck modeling of multicomponent ion transport in a Naflon membrane at high current density. J Appl Electrochem 47:51–62. https://doi.org/10.1007/s10800-016-1017-2
Neihof R, Sollner K (1957) The transitory overshooting of final equilibrium concentrations in membrane systems which drift toward the Gibbs-Donnan membrane equilibrium. J Phys Chem 61:159–163. https://doi.org/10.1021/j150548a008
Nguyen MK, Kurtz I (2006) Quantitative interrelationship between Gibbs-Donnan equilibrium, osmolality of body fluid compartments, and plasma water sodium concentration. J Appl Physiol 100:1293–1300. https://doi.org/10.1152/japplphysiol.01274.2005
Nouri S, Dammak L, Bulvestre G, Auclair B (2002) Studies of the crossed ionic fluxes through a cation-exchange membrane in the case of Donnan dialysis. Desalination 148:383–388. https://doi.org/10.1016/S0011-9164(02)00734-8
Osterhout WJV (1925) Is living protoplasm permeable to ions? J Gen Physiol 8:131–146. https://doi.org/10.1085/jgp.8.2.131
Osterhout WJV (1929) The kinetics of penetration. J Gen Physiol 13:261–294. https://doi.org/10.1085/jgp.13.2.261
Palmeri J, Lefebvre X (2006) Computer simulation of Nanofiltration, membranes and processes. In: Rieth M, Schommers W (eds) Handbook of theoretical and computational nanotechnology, Transport Phenomena and Nanoscale Processes, vol 5, 1st edn. American Scientific Publishers, Stevenson Ranch, pp 93–214
Philipse A, Vrij A (2011) The Donnan equilibrium: I. on the thermodynamic foundation of the Donnan equation of state. J Phys Condens Matter 23:194106. https://doi.org/10.1088/0953-8984/23/19/194106
Prado-Rubio OA, Møllerhøj M, Jørgensen SB, Jonsson G (2010) Modeling Donnan dialysis separation for carboxylic anion recovery. Comput Chem Eng 34:1567–1579. https://doi.org/10.1016/j.compchemeng.2010.03.003
Pyrzynska K (2006) Preconcentration and recovery of metal ions by Donnan dialysis. Microchim Acta 153:117–126. https://doi.org/10.1007/s00604-005-0434-4
Ramirez P, Alcaraz A, Mafe S, Pellicer J (2002) Donnan equilibrium of ionic drugs in pH-dependent fixed charge membranes: theoretical modelling. J Colloid Interface Sci 253:171–179. https://doi.org/10.1006/jcis.2002.8508
Rohman FS, Aziz N (2008) Mathematical model of ion transport in electrodialysis process. Bull Chem React Eng Catal 3:3–8. https://doi.org/10.9767/bcrec.3.1-3.7122.3-8
Sarkar S, Sengupta A, Prakash P (2010) The Donnan membrane principle: opportunities for sustainable engineered processes and materials. Environ Sci Technol 44:1161–1166. https://doi.org/10.1021/es9024029
Shu L, Liu X, Li Y, Yang B, Huang S, Lin Y, Jin S (2016) Modified Kedem-Katchalsky equations for osmosis through nano-pore. Desalination 399:47–52. https://doi.org/10.1016/j.desal.2016.08.011
Sobana S, Panda RC (2011) Review on modelling and control of desalination system using reverse osmosis. Rev Environ Sci Biotechnol 10:139–150. https://doi.org/10.1007/s11157-011-9233-z
Steele A, Arias J (2014) Accounting for the Donnan effect in diafiltration optimization for high concentration UFDF applications. BioProcess Int 12:50–54
Szczepański P, Szczepańska G (2017) Donnan dialysis − a new predictive model for non−steady state transport. J Membr Sci 525:277–289. https://doi.org/10.1016/j.memsci.2016.11.017
Tanaka Y (2012) Measurement of membrane characteristics using the phenomenological equation and the overall mass transport equation in ion-exchange membrane electrodialysis of saline water. Int J Chem Eng 2012:Article ID 148147, 12. https://doi.org/10.1155/2012/148147
Tian H, Zhang L, Wang M (2015) Applicability of Donnan equilibrium theory at nanochannel-reservoir interfaces. J Colloid Interface Sci 452:78–88. https://doi.org/10.1016/j.jcis.2015.03.064
Vega FA, Weng L, Temminghoff EJM, Van Riemsdijk WH (2010) Donnan membrane technique (DMT) for anion measurement. Anal Chem 82:2932–2939. https://doi.org/10.1021/ac9029339
Volpert AI, Hudyaev SI (1975) Analyses in classes of discontinuous functions and equations of mathematical physics. Nauka, Moscow. (In Russian)
Wang J, Dlamini DS, Mishra AK, Pendergast MTM, Wong MCY, Mamba BB, Freger V, Verliefde ARD, Hoek EMV (2014) A critical review of transport through osmotic membranes. J Membr Sci 454:516–537. https://doi.org/10.1016/j.memsci.2013.12.034
Yaroshchuk A, Martínez-Lladó X, Llenas L, Rovira M, de Pablo J (2011) Solution-diffusion-film model for the description of pressure-driven trans-membrane transfer of electrolyte mixtures: one dominant salt and trace ions. J Membr Sci 368:192–201. https://doi.org/10.1016/j.memsci.2010.11.037
Zhao R, Van Soestbergen M, Rijnaarts HHM, Van der Wal A, Bazant MZ, Biesheuvel PM (2012) Time-dependent ion selectivity in capacitive charging of porous electrodes. J Colloid Interface Sci 384:38–44. https://doi.org/10.1016/j.jcis.2012.06.022
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Appendix
Appendix
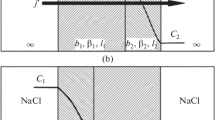
We will show that our kinetic model based on the assumption that only molecules (uncharged particles) penetrate through a membrane, and the Donnan model, based on the assumption that only ions can penetrate through a membrane, are equivalent. For this purpose, we use the “classical” thermodynamic approach and consider two states of the membrane system: the initial and equilibrium states.
In the initial state, the substance KB at concentration C1 and infinitely large dissociation constant K1, is located in the i-cell (i.e., only K+ and B− ions are present) while salt KA with concentration C2 and relatively small dissociation constant K2 is located in the e-cell. It should be taken into account that K+ and A− ions are in the i-cell simultaneously with the undissociated KA molecules. The cell volumes are equal to Vi = Ve.
To calculate the concentrations of ions and molecules in the e-cell, we designate z = [K+] = [A−] and write down an equation for the dissociation constant K2 (taking into account Eq. (8)):
Solving the quadratic equation in z, we get:
Thereby the initial state of the system under investigation may be presented as:
[K+]i = C1 | [K+]e = \( \frac{-{K}_2+\sqrt{K_2^2+4{K}_2{C}_2}}{2} \).
[B−]i = C1 | [A−]e = \( \frac{-{K}_2+\sqrt{K_2^2+4{K}_2{C}_2}}{2} \)
| [KA]e = \( \frac{2{C}_2+{K}_2-\sqrt{K_2^2+4{K}_2{C}_2}}{2} \)
Firstly, we will calculate the equilibrium state using the Donnan approach, i.e. we assume that only K+ and A− ions penetrate through the membrane.
As a result of permeable K+ and A− ions passing through the membrane, some amount of salt moves from the e-cell into the i-cell, taking into account that the electroneutrality condition requires transferring equal amounts of K+ and A− ions. As a result, the total salt concentration in the e-cell decreases by x and is then C2 – x. In accordance with Eq. (A1) in which C2 – x has to be written in the denominator instead of C2, it is possible to calculate the concentrations of ions and molecules in the e-cell at equilibrium.
Some of the ions that penetrate into the i-cell form molecules whose concentration is denoted by y. Then the concentrations of K+ cations and A− anions may be written as C1 + x – y and x – y, respectively. The concentrations of ions and undissociated molecules are related by the following equation:
with which y can be calculated:
Thereby the equilibrium state of the system under investigation may be presented as:
Now, let us assume that only undissociated KA molecules penetrate through the membrane like in our kinetic approach. In the process of transition of the system from the initial state to the equilibrium state, the total salt concentration in the e-cell will decrease by x, and these x moles of salt KA will move to the solution located in the i-cell. In this case, the concentrations of ions and molecules in the e-cell at equilibrium are as follows:
Some KA molecules that have moved to the i-cell dissociate into ions. Let us denote the concentration of resulting ions as y. Then the concentrations of K+ cations and A− anions may be written as C1 + y and y, respectively The concentrations of ions and undissociated molecules are related by the following equation:
with which y can be calculated:
The resulting expression is the concentration of the A− anion in i-cell ([A−]i). Now, it is possible to calculate [K+]i and [KA]i:
As you can see, both approaches lead to identical results, suggesting their equivalence. Thus, regardless of whether undissociated molecules or individual ions are transported through the membrane (in quantities guaranteeing the maintenance of electrical neutrality), the system reaches the same equilibrium state (from an identical initial state).
We also show that both approaches lead to the equality of equilibrium concentrations of undissociated salt molecules in solutions separated by a semipermeable membrane.
At equilibrium, the penetrating ions obey the Donnan distribution, so the following equations may be written:
Let us compare the concentrations of undissociated KA molecules in the i- and e-cells:
It is difficult to determine from Eq. (A13) if the above ratio equals one. So let us focus on Eqs. (A11) and (A12): their ratio equals one.
After transformations we get:
The comparison between Eqs. (A13) and (A15) clearly shows that at equilibrium the concentrations of undissociated molecules in the cells separated by a membrane are equal:
Rights and permissions
About this article
Cite this article
Karakhim, S.O., Zhuk, P.F. & Kosterin, S.O. Kinetics simulation of transmembrane transport of ions and molecules through a semipermeable membrane. J Bioenerg Biomembr 52, 47–60 (2020). https://doi.org/10.1007/s10863-019-09821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-019-09821-8