Abstract
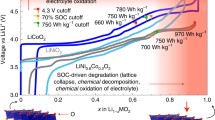
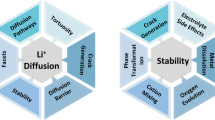
High-nickel layered oxide cathode materials will be at the forefront to enable longer driving-range electric vehicles at more affordable costs with lithium-based batteries. A continued push to higher energy content and less usage of costly raw materials, such as cobalt, while preserving acceptable power, lifetime and safety metrics, calls for a suite of strategic compositional, morphological and microstructural designs and efficient material production processes. In this Perspective, we discuss several important design considerations for high-nickel layered oxide cathodes that will be implemented in the automotive market for the coming decade. We outline various intrinsic restraints of maximizing their energy output and compare current/emerging development roadmaps approaching low-/zero-cobalt chemistry. Materials production is another focus, relevant to driving down costs and addressing the practical challenges of high-nickel layered oxides for demanding vehicle applications. We further assess a series of stabilization techniques on their prospects to fulfill the aggressive targets of vehicle electrification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Irle, R. Global EV sales for 2018 – final results. EV-volumes.com http://www.ev-volumes.com/news/global-ev-sales-for-2018/ (2019).
Global EV Outlook 2019 (International Energy Agency, 2019).
The Push to High Nickel Content in Layered Oxides – Production, Cost, and Supply & Demand (in Chinese) (Shanxi Securities, 2019).
Alvarez, S. Tesla model 3 battery details revealed in partial teardown and analysis. Teslarati https://www.teslarati.com/tesla-model-3-battery-details-partial-teardown-analysis (2018).
Goldie-Scot, L. A behind the scenes take on lithium-ion battery prices Bloomberg New Energy Finance https://about.bnef.com/blog/behind-scenes-take-lithium-ion-battery-prices/ (2019).
US DRIVE Electrochemical Energy Storage Technical Team Roadmap (US Council for Automotive Research, 2017).
Schmuch, R., Wagner, R., Hörpel, G., Placke, T. & Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Kwade, A. et al. Current status and challenges for automotive battery production technologies. Nat. Energy 3, 290–300 (2018).
Fang, Q. NCM/NCA vs. LFP: the Competitive Edge of LFP (in Chinese). China Battery Enterprise Alliance (March 2019).
China’s installed battery capacity surges to 56.9 GWh. InsideEVs https://insideevs.com/news/342285/chinas-installed-battery-capacity-surges-to-569-gwh/ (2019).
Pillot, C. Lithium Ion Battery Raw Materials Supply and Demand 2016–2025 (AABC Europe, 2017); http://cii-resource.com/cet/AABE-03-17/Presentations/BRMT/Pillot_Christophe.pdf
Myung, S.-T. et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS Energy Lett. 2, 196–223 (2017).
Kim, J. et al. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 8, 1702028 (2018).
Mineral Commodity Summaries 2018 (US Geological Survey, 2018).
Li, W., Yaghoobnejad Asl, H., Xie, Q. & Manthiram, A. Collapse of LiNi1-x-yCoxMnyO2 lattice at deep charge irrespective of Ni content in lithium-ion batteries. J. Am. Chem. Soc. 141, 5097–5101 (2019).
Wandt, J., Freiberg, A. T. S., Ogrodnik, A. & Gasteiger, H. A. Singlet oxygen evolution from layered transition metal oxide cathode materials and its implications for lithium-ion batteries. Mater. Today 21, 825–833 (2018).
Gilbert, J. A., Shkrob, I. A. & Abraham, D. P. Transition metal dissolution, ion migration, electrocatalytic reduction and capacity loss in lithium-ion full cells. J. Electrochem. Soc. 164, A389–A399 (2017).
Lin, F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014).
Ma, L., Nie, M., Xia, J. & Dahn, J. R. A systematic study on the reactivity of different grades of charged Li[NixMnyCoz]O2 with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Power Sources 327, 145–150 (2016).
Ohzuku, T., Ueda, A. & Nagayama, M. Electrochemistry and structural chemistry of LiNiO2 \((R\overline{3}{\rm{m}})\) for 4 volt secondary lithium cells. J. Electrochem. Soc. 140, 1862–1870 (1993).
You, Y., Celio, H., Li, J., Dolocan, A. & Manthiram, A. Modified high‐nickel cathodes with stable surface chemistry against ambient air for lithium‐ion batteries. Angew. Chem. Int. Ed. 57, 6480–6485 (2018).
Renfrew, S. E. & McCloskey, B. D. Residual lithium carbonate predominantly accounts for first cycle CO2 and CO outgassing of Li-stoichiometric and Li-rich layered transition-metal oxides. J. Am. Chem. Soc. 139, 17853–17860 (2017).
Yakovleva, M. From Raw Material to Next Generation Advanced Batteries (FMC Corporation, 2017).
Production and Cost Analysis of Nickel-Rich Layered Cathode Materials (in Chinese) (CITIC Securities, 2018).
Panasonic starts mass-production of high-capacity 3.1 Ah lithium-ion battery. Panasonic Newsroom Global https://news.panasonic.com/global/press/data/en091218-2/en091218-2.html (2009).
Jeong, M. H., Lee, J. H. & Jung, S. H. Electrolyte for lithium secondary battery and lithium secondary battery comprising same. US Patent 20,180,183,100 A1 (2018).
Vandeputte, K. Price elasticity of supply for cathode materials in a fast-growing vehicle electrification scenario. In Proc. Commercializing Advanced High-Energy Batteries for Heavy & Light EVs (AABC Europe, 2019).
Fiscal Year 2018 Advanced Vehicle Technologies Research Funding Opportunity Announcement (FOA): 1a. Developing Low-Cobalt Active Cathode Materials for Next-Generation Li-Ion Batteries. DOE DE-FOA-0001919 (US Department of Energy, 2018).
Delmas, C., Saadoune, I. & Rougier, A. The Cycling Properties of the LixNi1−yCoyO2 Electrode. J. Power Sources 44, 595–602 (1993).
Ohzuku, T., Ueda, A. & Kouguchi, M. Synthesis and characterization of LiAl1/4Ni3/4O2 \(({\rm{R}}\overline{3}{\rm{m}})\) for lithium‐ion (shuttlecock) batteries. J. Electrochem. Soc. 142, 4033–4039 (1995).
Guilmard, M., Rougier, A., Grüne, M., Croguennec, L. & Delmas, C. Effects of aluminum on the structural and electrochemical properties of LiNiO2. J. Power Sources 115, 305–314 (2003).
Arai, H., Okada, S., Sakurai, Y. & Yamaki, J. Electrochemical and thermal behavior of LiNi1−zMzO2 (M = Co, Mn, Ti). J. Electrochem. Soc. 144, 3117–3125 (1997).
Li, H. et al. Is cobalt needed in Ni-rich positive electrode materials for lithium ion batteries? J. Electrochem. Soc. 166, A429–A439 (2019).
Watanabe, S., Kinoshita, M., Hosokawa, T., Morigaki, K. & Nakura, K. Capacity fading of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (effect of depth of discharge in charge-discharge cycling on the suppression of the micro-crack generation of LiAlyNi1−x−yCoxO2 Particle). J. Power Sources 260, 50–56 (2014).
Li, W. et al. Mn versus Al in layered oxide cathodes in lithium‐ion batteries: a comprehensive evaluation on long‐term cyclability. Adv. Energy Mater. 8, 1703154 (2018).
Madhavi, S., Subba Rao, G. V., Chowdari, B. V. R. & Li, S. F. Y. Effect of aluminium doping on cathodic behaviour of LiNi0.7Co0.3O2. J. Power Sources 93, 156–162 (2001).
Rossen, E., Jones, C. D. W. & Dahn, J. R. Structure and electrochemistry of LixMnyNi1−yO2. Solid State Ion. 57, 311–318 (1992).
Seiwert, M. VW’s Batteries Contain Four Times as much Cobalt as Tesla Batteries (in German) (WirtschaftsWoche, 2019).
Fortuna, C. Cobalt-Free Car Batteries In the Works for Panasonic & Tesla. Clean Technica https://cleantechnica.com/2018/06/09/cobalt-free-car-batteries-in-the-works-for-panasonic-tesla/ (2018).
Lima, P. NIO Begins Deliveries of ES6 with NCM 811 Battery. PushEVs https://pushevs.com/2019/06/20/nio-begins-deliveries-of-es6-with-ncm-811-battery/ (2019).
Son, Y. S. & Hong, J. Method of fabricating cathode active material of lithium secondary battery. US Patent 20,180,034,050 A1 (2018).
Johnson Matthey achieves two major milestones in journey to commercialise eLNO. JM News https://matthey.com/news/2019/johnson-matthey-achieves-two-major-milestones-in-commercialisation-of-elno (2019).
Bianchini, M., Roca-Ayats, M., Hartmann, P., Brezesinski, T. & Janek, J. There and back again –the journey of LiNiO2 as a cathode active material. Angew. Chem. Int. Ed. 58, 2–27 (2019).
Weigel, T. et al. Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations. ACS Energy Lett. 4, 508–516 (2019).
Kim, U.-H. et al. Pushing the limit of layered transition metal oxide cathodes for high-energy density rechargeable Li ion batteries. Energy Environ. Sci. 11, 1271–1279 (2018).
Turcheniuk, K., Bondarev, D., Singhal, V. & Yushin, G. Ten years left to redesign lithium-ion batteries. Nature 31, 467–470 (2018).
Sun, Y.-K., Myung, S.-T., Kim, M.-H., Prakash, J. & Amine, K. Synthesis and characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the microscale core-shell structure as the positive electrode material for lithium batteries. J. Am. Chem. Soc. 127, 13411–13418 (2005).
Fiscal Year 1983 - 2019 SBIR/STTR Awards (US Small Business Administration, 2019).
Li, W., Kim, U.-H., Dolocan, A., Sun, Y.-K. & Manthiram, A. Formation and inhibition of metallic lithium microstructures in lithium batteries driven by chemical crossover. ACS Nano 11, 5853–5863 (2017).
Toya, H. N. et al. Nickel-cobalt-manganese complex hydroxide particles and method for producing same, positive electrode active material for nonaqueous electrolyte secondary battery and method for producing same, and nonaqueous electrolyte secondary battery. US Patent 20,120,270,107 A1 (2012).
Liu, Y., Zhu, Y. & Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 4, 540–550 (2019).
Muto, S. et al. Capacity-fading mechanisms of LiNiO2-based lithium-ion batteries: ii. diagnostic analysis by electron microscopy and spectroscopy. J. Electrochem. Soc. 156, A371–A377 (2009).
Orikasa, Y. et al. Ionic conduction in lithium ion battery composite electrode governs cross-sectional reaction distribution. Sci. Rep. 6, 26382 (2016).
Pullen, A., Rempel, J. & Sriramulu, S. Polycrystalline layered metal oxides comprising nano-crystals. US Patent 20,190,140,276 A1 (2019).
Kim, J. et al. Nickel-based active material for lithium secondary battery, method of preparing the same, and lithium secondary battery including positive electrode including the nickel-based active material. US Patent 20,180,026,268 A1 (2018).
Yan, P. et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 3, 600–605 (2018).
Li, J. et al. Comparison of single crystal and polycrystalline LiNi0.5Mn0.3Co0.2O2 positive electrode materials for high voltage Li-ion cells. J. Electrochem. Soc. 164, A1534–A1544 (2017).
Park, S., Chang, S. K., Park, H.-K., Hong, S. T. & Choi, Y. Electrode active material for lithium secondary battery. US Patent 20,140,356,719 A1 (2014).
Kimijima, T., Zettsu, N. & Teshima, K. Growth manner of octahedral-shaped Li(Ni1/3Co1/3Mn1/3)O2 single crystals in molten Na2SO4. Cryst. Growth Des. 16, 2618–2623 (2016).
Han, X., Meng, Q., Sun, T. & Sun, J. Preparation and electrochemical characterization of single-crystalline spherical LiNi1/3Co1/3Mn1/3O2 powders cathode material for Li-Ion Batteries. J. Power Sources 195, 3047–3052 (2010).
Kim, J. et al. A highly stabilized nickel-rich cathode material by nanoscale epitaxy control for high-energy lithium-ion batteries. Energy Environ. Sci. 11, 1449–1459 (2018).
Myung, S.-T. et al. Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. Chem. Mater. 17, 3695–3704 (2005).
Son, I. H., Park, J. H., Kwon, S., Mun, J. & Choi, J. W. Self-terminated artificial SEI Layer for nickel-rich layered cathode material via mixed gas chemical vapor deposition. Chem. Mater. 27, 7370–7379 (2015).
Maydannik, P. S., Kaariainen, T. O. & Cameron, D. C. Continuous atomic layer deposition: explanation for anomalous growth rate effects. J. Vac. Sci. Technol. A 30, 01A122 (2011).
Duong, H. M., Feigenbaum, H. & Hong, J. Dry energy storage device electrode and methods of making the same. US Patent, 20,150,303,481 A1 (2015).
Chiang, Y.-M., Duduta, M., Holman, R., Limthongkul, P. & Tan, T. Semi-solid electrodes having high rate capability. US Patent 20,140,170,524 A1 (2014).
Acknowledgements
The authors gratefully acknowledge the support from the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy through the award number DE-EE0008445 and the Welch Foundation F-1254.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have founded a startup company called TexPower to develop low-cobalt and cobalt-free cathode materials for lithium-based batteries.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, W., Erickson, E.M. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat Energy 5, 26–34 (2020). https://doi.org/10.1038/s41560-019-0513-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-019-0513-0
This article is cited by
-
Kirkendall effect-induced uniform stress distribution stabilizes nickel-rich layered oxide cathodes
Nature Communications (2024)
-
Re-evaluation of battery-grade lithium purity toward sustainable batteries
Nature Communications (2024)
-
Conversion of residual lithium into fast lithium ion conductor coating to achieve high cycle life LiNi0.8Co0.15Al0.05O2 cathode for lithium ion battery
Journal of Materials Science: Materials in Electronics (2024)
-
Structural effects of conductive additives on the molecular shuttles in lithium–organic batteries
Carbon Letters (2024)
-
Simulation of microalgae oil spray characteristics for mechanical fuel injection and CRDI systems
Biomass Conversion and Biorefinery (2024)