Abstract
Background
We evaluated: (1) associations of prenatal manganese (Mn) levels with child neurodevelopment at 4–6 years; (2) effect modification by maternal anemia and iron deficiency; and (3) sex-specific effects.
Methods
We measured blood Mn, hemoglobin, and serum ferritin in mothers at the second trimester, third trimester, and at birth, and in cord blood from a prospective birth cohort in Mexico City (n = 571). McCarthy Scales of Children’s Abilities were measured at 4–6 years. Using linear regression, we estimated associations between prenatal Mn and neurodevelopment, examined anemia and iron deficiency as effect modifiers, and analyzed associations by child sex.
Results
No direct associations were observed between Mn, anemia, or iron deficiency and McCarthy Scales. Second trimester iron deficiency and third trimester anemia modified the effect of Mn on child neurodevelopment. For instance, second trimester Mn was positively associated child memory scores in mother’s with normal ferritin (1.85 (0.02, 3.45)), but negatively associated in mother’s with low ferritin (−2.41 (−5.28, 0.47), interaction P value = 0.01), a pattern observed across scales. No effect modification at birth or in cord blood was observed.
Conclusions
Anemia/iron deficiency during pregnancy may modify Mn impacts on child neurodevelopment, particularly in boys.
Similar content being viewed by others
Introduction
Manganese (Mn) is an essential nutrient with a critical role in many cellular processes, including as calcium homeostasis, inflammatory response, and protein/energy metabolism.1 It is particularly important as a cofactor in the enzyme superoxide dismutase (SOD), which is crucial for protection against oxidative stress.1 Despite its contribution to brain development,2 high environmental Mn exposures have been implicated in developmental neurotoxicity, when Mn itself can induce oxidative stress. Mn is actively transferred across the placenta3 and across the developing blood–brain barrier to accumulate the neonate brain.4 Several studies have reported inverted U-shaped response curves for the effects of Mn on child mental and psychomotor development between 6 and 36 months of age, suggesting that both deficient and excess Mn can be neurotoxic.5,6,7 Others have observed inverse linear associations between prenatal Mn with child behavior in grade school.8 However, Mn levels in teeth were positively associated with memory, cognitive, and motor skills from 7 to 10.5 years.8 Thus, more research is necessary to determine the long-term effects of prenatal Mn on child cognition.
Mn also interacts with other metals to increase oxidative stress and neurotoxicity, including physiological interactions with iron. Mn accumulates during iron deficiency due to increased levels of shared transporters,6 which may increase Mn toxicity in the brain.9 In addition, particularly in situations of low iron stores, Mn can replace iron in the Fenton reaction to generate free radicals. This is notable as iron deficiency is a global public health issue and a major cause of anemia. The burden of iron deficiency is high in low- and middle-income countries, and is associated with many factors, including education, socioeconomic status (SES), infection, diet, and obesity.10,11,12 It is especially prevalent among pregnant women, since iron requirements triple during pregnancy.10 Prenatal iron deficiency anemia can decrease cognitive performance, growth, and energy in infants and children.10 Prenatal Mn was negatively associated with mental and psychomotor development in girls born to anemic mothers,5 suggesting that these two factors may interact to induce adverse neurodevelopmental effects.
Although studies have adjusted for hemoglobin or ferritin status as a covariate to isolate effects of prenatal Mn on child neurodevelopment and cognition,13,14 to our knowledge only one has examined the interactions between prenatal Mn and anemic status on child neurodevelopment up to 24 months.5 We hypothesized that prenatal anemia and/or iron deficiency would synergistically increase the neurotoxic effects of prenatal Mn in early childhood. Fetal development is a complex process that is particularly sensitive in comparison to later life stages. Furthermore, maternal Mn, hemoglobin, and iron levels vary substantially throughout pregnancy and at birth.10,15 Thus, our objective was to identify subpopulations of individuals that may have increased susceptibility to Mn-induced neurotoxicity and to address the role of critical exposure windows by examining the association of Mn measured in maternal blood from the second trimester, third trimester, and at birth, as well as Mn in cord blood, with child cognition and verbal, motor, perceptual performance, quantitative, and memory skills at 4 years of age using the McCarthy Scales of Children’s Abilities. We examine the effect modification of this relationship by maternal anemia and iron deficiency. Finally, we examine the sex specificity of these interactions. By interrogating effect modification of Mn by both serum ferritin and hemoglobin at multiple times throughout pregnancy, and incorporating the McCarthy Scales as a later neurodevelopmental indicator, we are able to advance the field beyond previous research5 and provide meaningful information on the complex interactions between prenatal Mn and anemia on child neurodevelopment.
Methods
Study population
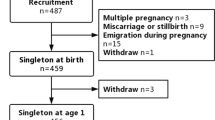
The Programming Research in Obesity, Growth Environment and Social Stress (PROGRESS) study is an ongoing, prospective, birth cohort in Mexico City, Mexico. We enrolled women receiving prenatal care from the Mexican Social Security Institute clinics before 20 weeks gestation as previously described,16,17 and 948 mother–child pairs were followed after birth. Smoking in pregnancy is uncommon in this population, but as it is associated with anemia18 and poorer neurodevelopment,19 six women reporting smoking during pregnancy were excluded. There were no differences between characteristics of the participants in this analysis with the whole study population. Procedures were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai, and the Mexican National Institute of Public Health. Women provided written informed consent.
Manganese measurement
Maternal blood was collected by trained research staff at the second trimester visit (12–34 weeks; n = 894), third trimester visit (26–38 weeks; n = 745), and at birth (maternal n = 732; cord n = 515) and stored at −20 °C. Mn was analyzed by inductively coupled plasma-mass spectrometry (ICP-MS/MS) as previously described for Pb with the appropriate quality controls.20 In brief, blood was digested in HNO3 and 30% H2O2 and analyzed via ICP-MS/MS on an Agilent 8800 ICP Triple Quad (ICP-QQQ) instrument (Agilent technologies Inc., Delaware, USA) with Indium as the internal standard. The quantitation limit of detection (LOD) was 0.02–0.08 µg/dL with no values below the LOD.
Blood parameter measurement
Hemoglobin was measured as part of a standard complete blood count (CBC) by trained research staff in blood from the second trimester visit, third trimester visit, in mothers at birth, and in cord blood. Serum was collected by centrifugation and ferritin was measured using an ELISA (enzyme-linked immunosorbent assay) chemiluminescence assay on an Immulite 100 (Siemens, Munich, Germany). Third trimester ferritin was measured on 586 of the 702 mothers with extra blood available as it was an addition to the standard CBC.
McCarthy’s Scales of children’s abilities
At the 4–6 years visit, the McCarthy’s Scales of Children’s Abilities examination was administered by a trained psychologist in Spanish21 at the Department of Developmental Neurobiology, National Institute of Perinatology, in Mexico City. The McCarthy Scales assess five key cognitive outcomes: memory, motor, perceptual performance (hereafter called “perception”), quantitative, and verbal skills. The perception, quantitative, and verbal scales were summed to create a General Cognitive Index (GCI). Raw values were standardized by child age. Subscales were scaled to a mean of 50 with a standard deviation of 10 and GCI was scaled to a mean of 100 with a standard deviation of 15.
Food Frequency Questionnaires for vitamin/supplement intake
Food Frequency Questionnaires (FFQs) validated for a Mexican population22 were administered to all participants at the third trimester and at birth for the previous 3 months (representing second and third trimester) in Spanish by trained research staff as previously described.23 Daily folate equivalents, iron intake, vitamin B12, thiamine, supplemental Mn, calcium, and daily β-carotene were estimated from FFQ supplement questionnaires as previously described in mg/day.23,24
Statistics
Mn values were trimmed to ±6 standard deviations to remove two extreme values from the third trimester. Hemoglobin levels are positively correlated with geographic elevation due to atmospheric oxygen tension. Thus, we corrected our values to reflect the high elevation of Mexico City (2250 m above sea level) by subtracting 1.3 g/dL from each measurement.25 We determined anemia status according to the World Health Organization guidelines for pregnancy for maternal measurements: 11 g/dL hemoglobin.10 Low ferritin/iron deficiency was considered <15 µg/L.10 Hemoglobin levels are highest at birth and fall to more typical levels following conversion of fetal hemoglobin to adult hemoglobin. There are currently no guidelines for anemia or iron deficiency in cord blood, so cord hemoglobin and iron were dichotomized at their respective medians for analysis. We determined differences between participant characteristics at each measurement time with mixed-effects models and calculated intraclass-correlation coefficients (ICCs) for Mn, hemoglobin, and ferritin values at all measurements and only in maternal measurements. We then used χ2, Fisher’s exact, or t tests to determine differences in participant characteristics stratified by each effect modifier.
We collected covariate information from second trimester baseline questionnaires. Covariates were selected for the model if they had a significant association with one of the cognitive outcomes or resulted in a change in the effect estimate of interest by >10% in the mutually adjusted models. Covariates considered for inclusion in the model were: maternal age, education, SES, marital status, parity, child age, sex, environmental tobacco smoke (estimate of secondhand smoke exposure as presence or absence of smokers at home), and date of visit (Supplementary Table S1 (online)). SES was calculated based on the AMAI (Mexican Association of Research and Public Opinion Agencies) rule 13 × 626 and then collapsed into a three-level index of low, medium, and high for this analysis. Although SES was associated with outcomes in univariate models, when education was also included, it did not alter the effect estimates of Mn. Thus, to preserve degrees of freedom in subpopulations with small sample sizes, SES was not included in our final adjusted models. Covariates included in adjusted models were: maternal education (<high school, high school, and >high school), maternal age, parity (primiparous or multiparous), child age, and sex.
We first assessed all associations between exposures and outcomes with generalized additive models to determine nonlinearity, which indicated linear associations (data not shown). Hence, we generated three linear regression models for the association between blood Mn at each measurement and scores on the McCarthy Scales: (1) models adjusted for hemoglobin or ferritin as covariates (Full); (2) models stratified by effect modifier status (low or normal hemoglobin/ferritin); and (3) models with an interaction between Mn and hemoglobin or ferritin. All associations were assessed in unadjusted models and models adjusted for all covariates. Effect estimates of the associations between Mn and child cognition can be interpreted as the change in McCarthy Scale per 1 µg/dL increase in Mn blood levels when Mn is measured in: all individuals, individuals with low ferritin/hemoglobin, or individuals with normal ferritin/hemoglobin. Effect estimates for the associations of effect modifier status on child cognition can be interpreted as the change in McCarthy Scale when the population changes from normal ferritin/hemoglobin to low ferritin/hemoglobin.
We also tested a three-way interaction between Mn, hemoglobin/ferritin, and child sex at each measurement time with child GCI. We fit three types of linear models: (1) models adjusted for hemoglobin or ferritin as a covariate and stratified by sex; (2) models stratified both by hemoglobin/ferritin and by sex; and (3) models containing a three-way interaction between Mn, hemoglobin or ferritin, and sex.
Finally, the use of daily vitamins may confound the relationships between Mn, anemia, and iron deficiency with neurodevelopment.23 We performed a sensitivity analysis including supplement use, stratifying all variables by their medians: daily folate equivalents (<850 mg/day, ≥850 mg/day), daily iron (<40 mg/day, ≥40 mg/day), vitamin B12 (None, >0), thiamine (None, >0); calcium (None, >0), and β-carotene supplementation (0, >600 mg/day) as covariates in our primary adjusted models. Separate models were specified for thiamine due to low numbers of participants taking thiamine supplements. The α-level for statistical tests of significance was set at 5%. We performed all statistical analyses in R version 3.5.0.27
Results
Population characteristics
Mothers in this study were primarily partnered (80.91%), multiparous (61.30%), and on average 27.57 years of age (Table 1). The majority were of low SES (51.66%), with 40.63% having less than a high school education. Children were split evenly between males and females. At the time of examination, children were on average 4.8 years old.
Whole-blood Mn concentrations increased from the second trimester to birth (Table 2). Serum ferritin levels decreased between the second and third trimesters, but returned to second trimester levels in mothers at birth. Maternal hemoglobin remained relatively constant from the second trimester to birth (Table 2). Mn, ferritin, and hemoglobin levels were greatest in cord blood. Reproducibility for Mn, ferritin, and hemoglobin measurements was null; however, when only maternal measurements were included, reproducibility was moderate (Table 2).
In the second trimester, 21% of mothers were anemic and 17% were iron deficient. In the third trimester, 23% of mothers were anemic and 54.4% were iron deficient. Finally, at birth, 33% of mothers were anemic and 21% were iron deficient. Among women with both hemoglobin and serum ferritin measured at the same time point, the majority of individuals had both high hemoglobin and ferritin (40–67%) depending on the trimester of measurement, while <20% had both values as low, and the remaining had either one low and one high measurement (28–42%) (Supplementary Table S2 (online)). Average Mn concentrations were higher in individuals with low ferritin at all measurements (Supplementary Table S3 (online)). When stratified by hemoglobin status, Mn concentrations were greater in the non-anemic population at the second trimester and in cord blood. The age of mothers with iron deficiency or with anemia in the third trimester was greater than mothers with normal iron or hemoglobin levels (~28 and 26 years, respectively). Mothers who were anemic in the second trimester tended to have lower BMI than non-anemic mothers (25.3 and 26.6 kg/m2, respectively), while mothers who were anemic at birth tended to have greater BMI than non-anemic mothers (26.03 and 27.07 kg/m2, respectively). Finally, boys had higher hemoglobin than girls in cord blood. No other covariates differed between low and normal populations (data not shown).
Associations of prenatal anemia and iron deficiency with child cognition
Neither low ferritin nor low hemoglobin was associated with child cognition, except second trimester low ferritin was positively associated with child motor scores in adjusted models (Fig. 1a). Mn was not independently associated with any of the McCarthy Scales in the full study population (un-stratified by hemoglobin or ferritin status) at any measurement (circles in Figs. 2 and 3). No differences were detected between adjusted and unadjusted models (Supplementary Fig. S1 (online)).
Effect modification of iron deficiency on the association between Mn measured in a second trimester, b third trimester, c cord blood, and d maternal blood at birth and McCarthy Scales in children at 4–6 years in adjusted models. Effect estimates with 95% confidence intervals (95% CI) are provided for the full population with ferritin status as a covariate (circle), in the subpopulation with low serum ferritin values (triangle), and the subpopulation with normal serum ferritin values (square). Plots are labeled with the interaction P value from adjusted models and statistically significant (p ≤ 0.05) interactions are indicated with an asterisk.
Effect modification of anemia on the association between Mn measured in a second trimester, b third trimester, c cord blood, and d maternal blood at birth and McCarthy Scales in children at 4–6 years in adjusted models. Effect estimates with 95% confidence intervals (95% CIs) are provided for the full population with hemoglobin status as a covariate (circle), in the subpopulation with low hemoglobin values (triangle), and the subpopulation with normal hemoglobin values (square). Plots are labeled with the interaction P value from adjusted models and statistically significant (p ≤ 0.05) interactions are indicated with an asterisk.
Associations of prenatal Mn with child cognition when stratified by serum ferritin
Second trimester Mn in mothers with normal ferritin was positively associated with child memory scores and quantitative scores (Fig. 2a). In contrast, in women with low ferritin, second trimester Mn exposure trended toward a negative association with GCI, memory, motor, quantitative, and verbal scores in children. Hence, ferritin status significantly modified the effect of second trimester maternal blood Mn on child memory scores (βnormal Fe = 1.85 (95% confidence interval (CI): 2.02, 3.45); βlow = −2.41 (−5.28, 0.47); adjusted interaction P (int. P) = 0.01) (Fig. 2a) and quantitative scores (βnormal Fe = 1.91 (0.12, 3.69); βlow = −2.13 (−5.51, 1.25); int. P = 0.05) (Fig. 2a). A similar trend of negative associations between Mn levels and cognition in low ferritin subgroups, and positive associations between Mn and cognitive outcomes in normal ferritin subgroups were present for children’s GCI, motor, and verbal scores (Fig. 2). When the sample was stratified by maternal ferritin status, no associations were observed between child cognition and blood Mn measured in the third trimester, in mothers at birth, or in cord blood (Fig. 2b–d). No differences were detected between adjusted and unadjusted models (Supplementary Fig. S2 (online))
Associations of prenatal Mn with child cognition when stratified by hemoglobin
When the sample was stratified by hemoglobin status, Mn in third trimester blood of mothers who were not anemic was positively associated with child memory scores at 4 years of age (Fig. 3b). Among anemic women, third trimester Mn levels trended toward inverse associations with child GCI, memory, motor, quantitative, and verbal scores. Anemia status in the third trimester significantly modified the effect of Mn on child’s GCI score (βnormal Hb = 1.64 (−0.19, 3.47); βlow = −2.68 (−6.77, 1.41); int. P = 0.05), child memory score (βnormal Hb = 1.34 (−0.17, 2.50); βlow = −1.38 (−4.05, 1.30); int. P = 0.05), and child verbal score (βnormal Hb = 1.17 (−0.07, 2.41); βlow = −1.51 (−4.21, 1.18); int. P = 0.05) (Fig. 3b). Furthermore, a trend toward this interaction was present for child motor and quantitative scores (Fig. 3b). No associations between Mn and child McCarthy scores were detected when Mn was measured in the second trimester, mothers at birth, or cord blood in samples stratified by anemic status (Fig. 3a, c, d). No differences were detected between adjusted and unadjusted models (Supplementary Fig. S3 (online)).
Sex-specific effects of prenatal Mn, anemia, and iron deficiency on child GCI
When the sample was stratified by child sex, no sex-specific associations of Mn with child GCI were detected or when the population was stratified by ferritin/hemoglobin in the second trimester, in cord blood, or in maternal blood at birth (Fig. 4).
Sex-specific effects on the interaction between Mn and ferritin or hemoglobin measured in a second trimester, b third trimester, c cord blood, and d maternal blood at birth and the general cognitive index (GCI) in boys (circles) and girls (triangles) at 4–6 years in adjusted models. Effect estimates with 95% confidence intervals (95% CI) are provided for the full population, the subpopulation with low values, and the subpopulation with normal values. Plots are labeled with the three-way interaction P value from adjusted models and statistically significant (p ≤ 0.05) interactions are indicated with an asterisk.
In the third trimester, no sex-specific associations between Mn and GCI were detected when the population was stratified by iron deficiency (Fig. 4b). However, a significant three-way interaction between third trimester anemia, Mn, and sex was observed for child GCI (int. P = 0.02). This effect was strongly observed in boys—third trimester maternal Mn from anemic mothers was inversely associated with GCI, but positively associated with GCI in non-anemic mothers (βnormal Hb boys = 2.50 (−0.25, 5.24); βlow boys = −5.55 (−12.33, 1.22)) (Fig. 4b). To evaluate the generalizability of our results, we ran these models with the remaining McCarthy Scales. A significant association was observed for child memory (Supplementary Fig. S4B (online)) and verbal scores (Supplementary Fig. S8B (online)), with similar trend for quantitative scores (Supplementary Fig. S7B (online)).
Sensitivity analysis: adjustment for second and third trimester supplement use
Generally, supplement use differed between low and normal populations of hemoglobin tin the second trimester and between low and normal populations of serum ferritin in the third trimester (Supplementary Table S4 (online)). Adjustment of second and third trimester models for any vitamins or supplements did not change the associations of anemic factors or of Mn with child McCarthy Scales (Supplementary Figs. 9 and S10 (online)).
Discussion
We examined the associations between Mn measured throughout pregnancy with child cognition and assessed effect modification by concurrent anemia and iron deficiency. Although we detected no direct effect of Mn or anemia/iron deficiency measured at any time point on child neurodevelopment outcomes in the full, un-stratified, population, we observed significant interactions for second trimester maternal blood Mn by second trimester ferritin status, and of third trimester maternal Mn by third trimester hemoglobin status on child GCI, memory, motor, quantitative, and verbal scores. Our results were consistent with the nutritional properties of both elements—Mn was beneficial when iron status was within normal limits, but associated with lower McCarthy Scale scores in the setting of anemia or iron deficiency. We also observed a effect modification by third trimester anemic status in boys on GCI status. These results suggest that iron and Mn status may act in tandem on the developing nervous system and that deficiency in one of the elements can impact the benefits of the other.
Research suggests that high prenatal Mn exposure adversely effects child neurodevelopment. In an analysis in this cohort at 24 months, researchers observed a significant association of third trimester and cord blood Mn with decreased cognitive, language, and motor development.7 Furthermore, in other cohorts, prenatal Mn exposure was adversely associated with cognitive and language scores at 2 years28 and cord blood Mn levels were negatively associated with three McCarthy scores at 3 years, but not at 6 years.29 Other studies have found associations of prenatal Mn with child behavioral problems.8
The primary mechanisms of Mn neurotoxicity are not well understood, but may involve increased oxidative damage to neuronal cells with targeting of glutaminergic and dopaminergic systems impacting executive function and attention.30 The adverse effects of iron deficiency are also mediated in part by dopaminergic neurons, as iron deficiency anemia increases spontaneous dopamine release in the nucleus accumbens among other effects on dopamine pathways,31 as well as glutaminergic systems and myelination.32 Glutamate and dopamine are essential to learning and behavioral development, suggesting that the combination of higher Mn and lower iron status may be operating via dopaminergic/glutaminergic pathways that are shared by iron and Mn. In this population, second and third trimester Mn trended towards being inversely associated with child cognition in mothers with low second or third trimester ferritin or hemoglobin. In contrast, second and third trimester Mn in mothers with normal ferritin and hemoglobin was positively associated with child cognition.
Interestingly, effect modification of the association between Mn and child cognition by iron deficiency was observed in the second trimester and effect modification by anemia was observed in the third trimester. These results suggest that earlier development is more susceptible to the interactions between Mn and anemia or iron deficiency. Research demonstrates that fetal development is more vulnerable than postnatal development to toxicant exposure. This may be due to differing physiological requirements for Mn or iron by developmental stage, or by changing pharmacokinetics throughout development, particularly in terms of drug metabolizing enzymes, and hepatic and renal clearance systems.33 Additionally, although Mn can cross the blood–brain barrier, it has been suggested that incomplete blood–brain barrier development may increase Mn uptake earlier in pregnancy.4 Furthermore, the transferrin receptor is expressed early in development throughout the brain, possibly explaining increased iron uptake.4 Mn is also able to bind to transferrin for transport via neuronal divalent metal transporter,4 suggesting that in situations of low iron, Mn uptake may increase due to decreased binding competition. When measured in the third trimester, the effects of Mn were modified only by anemia and not iron deficiency. However, ~75% of cases of anemia during pregnancy are due to iron deficiency.34 In this instance, it is possible that the anemic state measured during the third trimester is a better reflection of iron deficiency anemia than the serum ferritin measurement. Thus, effect modification similar to second trimester Mn and iron deficiency is now detectable as an interaction between third trimester Mn and anemia.
We also observed effect modification by sex and third trimester hemoglobin on Mn and child GCI scores, which was also apparent in memory, quantitative, and verbal scores. A negative relationship between Mn and neurodevelopment was detected in boys born to mothers with low third trimester hemoglobin. To date, only one other study has investigated a three-way interaction between Mn, hemoglobin, and cognition. In 6-month-old infants, Gunier et al.5 observed that girls born to mothers with low hemoglobin had lower mental and psychomotor development indexes than those born to mothers with higher hemoglobin. They observed no effects in boys. Previous studies have reported that girls tend to have higher Mn blood levels than boys,5,8 and cross-sectional analyses in children have reported adverse effects on neurodevelopment in girls.35 In contrast, we observed no effect modification in girls. However, another study found a negative association between cord blood Mn and hand skills in boys, but not in girls.29
The Mn levels measured in this study were lower than previously assessed in maternal whole blood in the United States,36 approximately equal to concentrations in South Africa,37 but higher than those in France.38 However, the timing of the Mn measurements during pregnancy may differ across studies, complicating direct comparisons. We observed an increase in Mn in maternal blood between the second trimester, third trimester, and at birth (ICC = 0.27), which is expected, as Mn concentrates during pregnancy to provide the fetus with adequate supply.15 Population average Mn measured in cord blood was approximately double Mn measured in maternal blood at birth, which is comparable to previous research.15,36,37 However, ICCs including cord blood were null, and low correlations between maternal Mn at birth and cord blood Mn have been reported previously.
Approximately 20% of women in our population were anemic during pregnancy, which is expected within the Mexican population.39 Ferritin levels in this population decreased by half between the second and third trimesters, as nutritional iron requirements triple through pregnancy.10 However, serum ferritin levels in mothers at birth were similar to those measured during the second trimester, which may be due to iron supplementation during pregnancy. Mn levels were significantly higher in iron deficient mothers, which is consistent with well-established interactions between Mn and Fe.6,40
We also observed differences in maternal BMI by anemic status and by trimester. Women that were anemic in the second trimester tended to have lower BMI, whereas women who were anemic at birth tended to have higher BMI than women with normal hemoglobin. Previous research suggests that obesity contributes to iron deficiency in pregnancy.11 It is possible that the effect of obesity on anemia may be small early in pregnancy, but as physiological iron requirements increase, the effects of obesity on iron deficiency become apparent. While we were unable to test this hypothesis, this pattern deserves future investigation.
This study has several limitations. First, Mn measured in blood does not indicate chronic exposure, however, whole-blood Mn is regarded as a useful biomarker of exposure and multiple studies report associations between prenatal blood Mn and neurodevelopment. Second, maternal Mn levels may underestimate prenatal child exposures because Mn is known to concentrate in the developing fetus.3 Finally, the small sample size for serum ferritin measurements limits our ability to detect three-way interactions with Mn, ferritin, and child sex through the lack of statistical power or potential increase in bias.
Nonetheless, our analysis also had several strengths, including prospective follow-up with high retention and multiple measurements of Mn during pregnancy. Moreover, we analyzed both ferritin and hemoglobin as effect modifiers. Although serum ferritin is also increased during inflammation, it is a more stable indicator of iron status than blood iron levels. We also demonstrated the robustness of our results by including use of several supplements relevant for anemia and neurodevelopment as covariates in our sensitivity analyses. This study is one of few to examine prenatal Mn in relation to neurodevelopment in children over 36 months of age8 and to examine the McCarthy Scales of Children’s Abilities as the outcome. Future studies would benefit from longitudinal analysis of child neurodevelopment and exploration of the relationship between childhood Mn and anemia.
Conclusion
In this population, we observed effect modification of the association between prenatal Mn and child neurodevelopment at 4–6 years by maternal anemia and iron deficiency. Specifically, positive associations were observed between Mn and child cognition, verbal, memory, motor, and quantitative scores among children whose mothers had normal hemoglobin or ferritin in the second and third trimesters. In contrast, associations between neurodevelopment and Mn were negative in children born to mothers that were anemic in the third trimester or iron deficient in the second trimester. This effect was most pronounced in boys with mothers that were anemic in the third trimester of pregnancy. No associations were observed when Mn and hemoglobin/ferritin were measured in maternal blood at birth or in cord blood. Additional research on the timing of exposure in relation to the central nervous system is needed to fully elucidate these effects.
References
Freeland-Graves, J. H., Mousa, T. Y., Sanjeevi, N. in Manganese in Health and Disease 34–75 (2014). http://pubs.rsc.org/en/content/chapter/bk9781849739436-00034/978-1-84973-943-6
Hurley, L. S. The role of trace elements in foetal and neonatal development. Philos. Trans. R. Soc. Lond. Ser. B 294, 145–152 (1981).
Shaw, J. C. Trace metal requirements of preterm infants. Acta Paediatr. Scand. Suppl. 296, 93–100 (1982).
Ek, C. J., Dziegielewska, K. M., Habgood, M. D. & Saunders, N. R. Barriers in the developing brain and neurotoxicology. Neurotoxicology 33, 586–604 (2012).
Gunier, R. B. et al. Manganese in teeth and neurodevelopment in young Mexican–American children. Environ. Res. 142, 688–695 (2015).
Henn, B. C. et al. Early postnatal blood manganese levels and children’s neurodevelopment. Epidemiology 21, 433–439 (2010).
Muñoz-Rocha, T. V. et al. Prenatal co-exposure to manganese and depression and 24-months neurodevelopment. Neurotoxicology (2017). http://www.sciencedirect.com/science/article/pii/S0161813X17301201
Mora, A. M. et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ. Int. 84, 39–54 (2015).
Seo, Y. A., Li, Y. & Wessling-Resnick, M. Iron depletion increases manganese uptake and potentiates apoptosis through ER stress. Neurotoxicology 38, 67–73 (2013).
World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide For Programme Managers (WHO, Geneva, Switzerland, 2001).
Garcia-Valdes, L. et al. The impact of maternal obesity on iron status, placental transferrin receptor expression and hepcidin expression in human pregnancy. Int. J. Obes. 39, 571–578 (2015).
Balarajan, Y., Ramakrishnan, U., Özaltin, E., Shankar, A. H. & Subramanian, S. V. Anaemia in low-income and middle-income countries. Lancet 378, 2123–2135 (2011).
Claus Henn, B et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site. Environ. Health Perspect. (2017). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5743453/
Henn, B. C. et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ. Health Perspect. 120, 126–131 (2012).
Krachler, M., Rossipal, E. & Micetic-Turk, D. Trace element transfer from the mother to the newborn—investigations on triplets of colostrum, maternal and umbilical cord sera. Eur. J. Clin. Nutr. 53, 486–494 (1999).
Braun, J. M. et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from Mexico City: a cross-sectional study. Environ. Health 13, 50 (2014).
Burris, H. H. et al. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 5, 271–281 (2013).
Leifert, J. A. Anaemia and cigarette smoking. Int. J. Lab. Hematol. 30, 177–184 (2008).
Wehby, G. L., Prater, K., McCarthy, A. M., Castilla, E. E. & Murray, J. C. The impact of maternal smoking during pregnancy on early child neurodevelopment. J. Hum. Cap. 5, 207–254 (2011).
Renzetti, S. et al. The association of lead exposure during pregnancy and childhood anthropometry in the Mexican PROGRESS cohort. Environ. Res. 152, 226–232 (2017).
McCarthy, D. Manual for the McCarthy Scales of Children’s Abilities. New York, Psychological Corporation, 1972.
Hernández-Avila, M. et al. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Sal. Pub. Mex. 40, 133–140 (1998).
Malin, A. J. et al. Quality of prenatal and childhood diet predicts neurodevelopmental outcomes among children in Mexico City. Nutrients (2018). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6115750/
Rodríguez-Ramírez, S., Mundo-Rosas, V., Jiménez-Aguilar, A. & Shamah-Levy, T. Methodology for the analysis of dietary data from the Mexican National Health and Nutrition Survey 2006. Sal. Pub. Mex. 51(Suppl. 4), S523–S529 (2009).
Dirren, H., Logman, M. H., Barclay, D. V. & Freire, W. B. Altitude correction for hemoglobin. Eur. J. Clin. Nutr. 48, 625–632 (1994).
Carrasco, A. V. The AMAI System of Classifying Households by Socio-economic Level: The experience of Mexico and its comparison with Brazil and Argentina (ESOMAR, 2002).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2018). https://www.R-project.org/
Lin, C.-C. et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ. Res. 123, 52–57 (2013).
Takser, L., Mergler, D., Hellier, G., Sahuquillo, J. & Huel, G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology 24, 667–674 (2003).
Aschner, M. & Aschner, J. L. Manganese neurotoxicity: cellular effects and blood-brain barrier transport. Neurosci. Biobehav. Rev. 15, 333–340 (1991).
Nelson, C., Erikson, K., Piñero, D. J. & Beard, J. L. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J. Nutr. 127, 2282–2288 (1997).
Kim, J. & Wessling-Resnick, M. Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 25, 1101–1107 (2014).
Alcorn, J. & McNamara, P. J. Ontogeny of hepatic and renal systemic clearance pathways in infants. Part I. Clin. Pharmacokinet. 41, 959–998 (2002).
Di Renzo, G. C. et al. Iron deficiency anemia in pregnancy. Women’s Health (Lond. Engl.) 11, 891–900 (2015).
Menezes-Filho, J. A. et al. Elevated manganese exposure and school-aged children’s behavior: a gender-stratified analysis. Neurotoxicology 45, 293–300 (2014).
Zota, A. R. et al. Maternal blood manganese levels and infant birth weight. Epidemiology 20, 367–373 (2009).
Rudge, C. V. et al. The placenta as a barrier for toxic and essential elements in paired maternal and cord blood samples of South African delivering women. J. Environ. Monit. 11, 1322–1330 (2009).
Abdelouahab, N. et al. Monoamine oxidase activity in placenta in relation to manganese, cadmium, lead, and mercury at delivery. Neurotoxicol. Teratol. 32, 256–261 (2010).
Shamah-Levy, T. et al. Tendencia en la prevalencia de anemia entre mujeres mexicanas en edad reproductiva 2006-2016. Ensanut MC 2016. Sal. Púb. Méx. 60, 301–308 (2018).
Finley, J. W. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 70, 37–43 (1999).
Acknowledgements
We thank the American British Cowdray Hospital in Mexico City for providing research facilities. Work was supported by NIH grants: R01ES014930; R01ES013744; R01ES021357, P30ES009089, P30ES023515, and R24ES028522. Co-investigators at the INSP received partial funding from the National Institute of Public Health/Ministry of Health of Mexico.
Author information
Authors and Affiliations
Contributions
A.K. conceived the study design, performed the data analysis, interpreted the results, and drafted the manuscript. G.E.-G., A.C., L.S., I.P., C.A., and K.S. provided substantial contributions to data acquisition. D.C.B., M.M.T.-R., A.A.B., and R.O.W., each contributed substantially to study desgin and data interpretation, and critically revised the manuscript intelectual content. All authors provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Kupsco, A., Estrada-Gutierrez, G., Cantoral, A. et al. Modification of the effects of prenatal manganese exposure on child neurodevelopment by maternal anemia and iron deficiency. Pediatr Res 88, 325–333 (2020). https://doi.org/10.1038/s41390-020-0754-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0754-4
This article is cited by
-
Associations of intrauterine exposure to manganese with fetal and early-childhood growth: a prospective prenatal cohort study
Environmental Science and Pollution Research (2024)
-
Adapting the preterm birth phenotyping framework to a low-resource, rural setting and applying it to births from Migori County in western Kenya
BMC Pregnancy and Childbirth (2023)
-
Environmental Metal Exposure, Neurodevelopment, and the Role of Iron Status: a Review
Current Environmental Health Reports (2022)