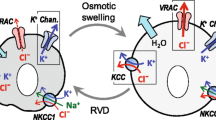
Abstract
The solute carrier 8 (SLC8) family of sodium–calcium exchangers (NCXs) functions as an essential regulatory system that couples opposite fluxes of sodium and calcium ions across plasmalemmal membranes. NCXs, thereby, play key roles in maintaining an ion homeostasis that preserves cellular integrity. Hence, alterations in NCX expression and regulation have been found to lead to ionic imbalances that are often associated with intracellular calcium overload and cell death. On the other hand, intracellular calcium has been identified as a key driver for a multitude of downstream signaling events that are crucial for proper functioning of biological systems, thus highlighting the need for a tightly controlled balance. In the CNS, NCXs have been primarily characterized in the context of synaptic transmission and ischemic brain damage. However, a much broader picture is emerging. NCXs are expressed by virtually all cells of the CNS including oligodendrocytes (OLGs), the cells that generate the myelin sheath. With a growing appreciation of dynamic calcium signals in OLGs, NCXs are becoming increasingly recognized for their crucial roles in shaping OLG function under both physiological and pathophysiological conditions. In order to provide a current update, this review focuses on the importance of NCXs in cells of the OLG lineage. More specifically, it provides a brief introduction into plasmalemmal NCXs and their modes of activity, and it discusses the roles of OLG expressed NCXs in regulating CNS myelination and in contributing to CNS pathologies associated with detrimental effects on OLG lineage cells.




Similar content being viewed by others
References
Kawamoto EM, Vivar C, Camandola S (2012) Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3:61. https://doi.org/10.3389/fphar.2012.00061
Brini M, Calì T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71:2787–2814. https://doi.org/10.1007/s00018-013-1550-7
Horigane S-I, Ozawa Y, Yamada H, Takemoto-Kimura S (2019) Calcium signalling: a key regulator of neuronal migration. J Biochem 165:401–409. https://doi.org/10.1093/jb/mvz012
Toth AB, Shum AK, Prakriya M (2016) Regulation of neurogenesis by calcium signaling. Cell Calcium 59:124–134. https://doi.org/10.1016/j.ceca.2016.02.011
Shigetomi E, Patel S, Khakh BS (2016) Probing the complexities of astrocyte calcium signaling. Trends Cell Biol 26:300–312. https://doi.org/10.1016/j.tcb.2016.01.003
Verkhratsky A, Untiet V, Rose CR (2019) Ionic signalling in astroglia beyond calcium. J Physiol. https://doi.org/10.1113/JP277478
Färber K, Kettenmann H (2006) Functional role of calcium signals for microglial function. Glia 54:656–665. https://doi.org/10.1002/glia.20412
Brawek B, Garaschuk O (2013) Microglial calcium signaling in the adult, aged and diseased brain. Cell Calcium 53:159–169. https://doi.org/10.1016/j.ceca.2012.12.003
Zhang M, Liu Y, Wu S, Zhao X (2019) Ca2+ Signaling in oligodendrocyte development. Cell Mol Neurobiol 39:1071–1080. https://doi.org/10.1007/s10571-019-00705-4
Pitman KA, Young KM (2016) Activity-dependent calcium signalling in oligodendrocyte generation. Int J Biochem Cell Biol 77:30–34. https://doi.org/10.1016/j.biocel.2016.05.018
Butt AM (2006) Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia 54:666–675. https://doi.org/10.1002/glia.20424
Soliven B (2001) Calcium signalling in cells of oligodendroglial lineage. Microsc Res Tech 15:672–679
Cheli VT, Santiago González DA, Spreuer V, Paez PM (2015) Voltage-gated Ca++ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp Neurol 265:69–83. https://doi.org/10.1016/j.expneurol.2014.12.012
Burgoyne RD, Helassa N, McCue HV, Haynes LP (2019) Calcium sensors in neuronal function and dysfunction. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a035154
Schrank S, Barrington N, Stutzmann GE (2019) Calcium-handling defects and neurodegenerative disease. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a035212
Berridge MJ (2014) Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res 357:477–492. https://doi.org/10.1007/s00441-014-1806-z
Shigetomi E, Saito K, Sano F, Koizumi S (2019) Aberrant CalciuASN Neuro m signals in reactive astrocytes: a key process in neurological disorders. Int J Mol Sci. https://doi.org/10.3390/ijms20040996
Verkhratsky A, Rodríguez-Arellano JJ, Parpura V, Zorec R (2017) Astroglial calcium signalling in Alzheimer’s disease. Biochem Biophys Res Commun 483:1005–1012. https://doi.org/10.1016/j.bbrc.2016.08.088
Nedergaard M, Rodríguez JJ, Verkhratsky A (2010) Glial calcium and diseases of the nervous system. Cell Calcium 47:140–149. https://doi.org/10.1016/j.ceca.2009.11.010
Blaustein MP, Lederer WJ (1999) Sodium/calcium exchange: its physiological implications. Physiol Rev 79:763–854
DiPolo Reinaldo, Beauge L (1987) Plasma membrane mechanisms for intracellular calcium regulation in squid axons. Acta Physiol Pharmacol Latinoam 37:437–444
Khananshvili D (1990) Distinction between the two basic mechanisms of cation transport in the cardiac Na(+)-Ca2+ exchange system. Biochemistry 29:2437–2442. https://doi.org/10.1021/bi00462a001
Reeves JP, Condrescu M, Chernaya G, Gardner JP (1994) Na+/Ca2+ antiport in the mammalian heart. J Exp Biol 196:375–388
Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA (1969) The influence of calcium on sodium efflux in squid axons. J Physiol 200:431–458. https://doi.org/10.1113/jphysiol.1969.sp008702
Reyes RC, Verkhratsky A, Parpura V (2012) Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. https://doi.org/10.1042/AN20110059
Parpura V, Sekler I, Fern R (2016) Plasmalemmal and mitochondrial Na(+) -Ca(2 +) exchange in neuroglia. Glia 64:1646–1654. https://doi.org/10.1002/glia.22975
Roome CJ, Power EM, Empson RM (2013) Transient reversal of the sodium/calcium exchanger boosts presynaptic calcium and synaptic transmission at a cerebellar synapse. J Neurophysiol 109:1669–1680. https://doi.org/10.1152/jn.00854.2012
Jeon D, Yang Y-M, Jeong M-J et al (2003) Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38:965–976. https://doi.org/10.1016/s0896-6273(03)00334-9
Molinaro P, Viggiano D, Nisticò R et al (2011) Na+–Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long-term potentiation and spatial learning and memory. J Neurosci 31:7312–7321. https://doi.org/10.1523/JNEUROSCI.6296-10.2011
Molinaro P, Cuomo O, Pignataro G et al (2008) Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci 28:1179–1184. https://doi.org/10.1523/JNEUROSCI.4671-07.2008
Pignataro G, Sirabella R, Anzilotti S et al (2014) Does Na+/Ca2+ exchanger, NCX, represent a new druggable target in stroke intervention? Transl Stroke Res 5:145–155. https://doi.org/10.1007/s12975-013-0308-8
Jeffs GJ, Meloni BP, Sokolow S et al (2008) NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp Neurol 210:268–273. https://doi.org/10.1016/j.expneurol.2007.10.013
Sirabella R, Sisalli MJ, Costa G et al (2018) NCX1 and NCX3 as potential factors contributing to neurodegeneration and neuroinflammation in the A53T transgenic mouse model of Parkinson’s Disease. Cell Death Dis 9:725. https://doi.org/10.1038/s41419-018-0775-7
Takuma K, Ago Y, Matsuda T (2013) The glial sodium-calcium exchanger: a new target for nitric oxide-mediated cellular toxicity. Curr Protein Pept Sci 14:43–50
Noda M, Ifuku M, Mori Y, Verkhratsky A (2013) Calcium influx through reversed NCX controls migration of microglia. Advances in experimental medicine and biology. Springer, Boston, pp 289–294
Boscia F, Gala R, Pannaccione A et al (2009) NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke 40:3608–3617. https://doi.org/10.1161/STROKEAHA.109.557439
Khananshvili D (2013) The SLC8 gene family of sodium-calcium exchangers (NCX)—structure, function, and regulation in health and disease. Mol Aspects Med 34:220–235. https://doi.org/10.1016/j.mam.2012.07.003
Lytton J (2007) Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J 406:365–382. https://doi.org/10.1042/BJ20070619
Carafoli E (1987) Intracellular calcium homeostasis. Annu Rev Biochem 56:395–433. https://doi.org/10.1146/annurev.bi.56.070187.002143
Philipson KD, Nicoll DA (2000) Sodium–calcium exchanger: a molecular perspective. Annu Rev Physiol 62:111–133. https://doi.org/10.1146/annurev.physiol.62.1.111
Nicoll D, Longoni S, Philipson K (1990) Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)-Ca2 + exchanger. Science 250:562–565. https://doi.org/10.1126/science.1700476
Li Z, Matsuokas S, Hryshkob LV et al (1994) Cloning of the NCX2 isoform of the plasma membrane Na+–Ca2+ exchanger. J Biol Chem 269:17434–17439
Nicoll DA, Quednau BD, Qui Z et al (1996) Cloning of a third mammalian Na+–Ca2+ exchanger, NCX3. J Biol Chem 271:24914–24921. https://doi.org/10.1074/jbc.271.40.24914
On C, Marshall CR, Perry SF et al (2009) Characterization of zebrafish (Danio rerio) NCX4: a novel NCX with distinct electrophysiological properties. Am J Physiol Physiol 296:C173–C181. https://doi.org/10.1152/ajpcell.00455.2008
On C, Marshall CR, Chen N et al (2008) Gene structure evolution of the Na+–Ca2+ exchanger (NCX) family. BMC Evol Biol 8:127. https://doi.org/10.1186/1471-2148-8-127
Marshall CR, Fox JA, Butland SL et al (2005) Phylogeny of Na +/Ca2 + exchanger (NCX) genes from genomic data identifies new gene duplications and a new family member in fish species. Physiol Genomics 21:161–173. https://doi.org/10.1152/physiolgenomics.00286.2004
Boyman L, Williams GSB, Khananshvili D et al (2013) NCLX: the mitochondrial sodium calcium exchanger. J Mol Cell Cardiol 59:205–213
Palty R, Silverman WF, Hershfinkel M et al (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA 107:436–441. https://doi.org/10.1073/pnas.0908099107
Palty R, Sekler I (2012) The mitochondrial Na+/Ca2+ exchanger. Cell Calcium 52:9–15
Linck B, Qiu Z, He Z et al (1998) Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am J Physiol 274:C415–C423. https://doi.org/10.1152/ajpcell.1998.274.2.C415
Ren X, Philipson KD (2013) The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J Mol Cell Cardiol 57:68–71. https://doi.org/10.1016/j.yjmcc.2013.01.010
Liao J, Li H, Zeng W et al (2012) Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335:686–690. https://doi.org/10.1126/science.1215759
Liao J, Marinelli F, Lee C et al (2016) Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger. Nat Struct Mol Biol 23:590–599. https://doi.org/10.1038/nsmb.3230
Giladi M, Shor R, Lisnyansky M, Khananshvili D (2016) Structure-functional basis of ion transport in sodium–calcium exchanger (NCX) proteins. Int J Mol Sci 17:1949. https://doi.org/10.3390/ijms17111949
Jardetzky O (1966) Simple allosteric model for membrane pumps. Nature 211:969–970. https://doi.org/10.1038/211969a0
Forrest LR, Krämer R, Ziegler C (2011) The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807:167–188. https://doi.org/10.1016/j.bbabio.2010.10.014
Khananshvili D (2014) Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers Arch 466:43–60. https://doi.org/10.1007/s00424-013-1405-y
Giladi M, Hiller R, Hirsch JA, Khananshvili D (2013) Population shift underlies Ca2+-induced regulatory transitions in the sodium-calcium exchanger (NCX). J Biol Chem 288:23141–23149. https://doi.org/10.1074/jbc.M113.471698
Ottolia M, Nicoll DA, John S, Philipson KD (2010) Interactions between Ca2+ binding domains of the Na+–Ca2+ exchanger and secondary regulation. Channels 4:159–162. https://doi.org/10.4161/chan.4.3.11386
Giladi M, Lee SY, Ariely Y et al (2017) Structure-based dynamic arrays in regulatory domains of sodium-calcium exchanger (NCX) isoforms. Sci Rep 7:993. https://doi.org/10.1038/s41598-017-01102-x
Hilge M, Aelen J, Vuister GW (2006) Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol Cell 22:15–25. https://doi.org/10.1016/j.molcel.2006.03.008
Besserer GM, Ottolia M, Nicoll DA et al (2007) The second Ca2 + -binding domain of the Na + -Ca 2 + exchanger is essential for regulation: crystal structures and mutational analysis. Proc Natl Acad Sci U S A 104:18467–18472. https://doi.org/10.1073/pnas.0707417104
Hilgemann DW, Matsuoka S, Nagel GA, Collins A (1992) Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol 100:905–932. https://doi.org/10.1085/jgp.100.6.905
Hilge M (2013) Ca(2 +) regulation in the Na(+)/Ca (2 +) exchanger features a dual electrostatic switch mechanism. Adv Exp Med Biol 961:27–33. https://doi.org/10.1007/978-1-4614-4756-6_3
Hilge M (2012) Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger. J Biol Chem 287:31641–31649. https://doi.org/10.1074/jbc.R112.353573
Tal I, Kozlovsky T, Brisker D et al (2016) Kinetic and equilibrium properties of regulatory Ca2 + -binding domains in sodium–calcium exchangers 2 and 3. Cell Calcium 59:181–188. https://doi.org/10.1016/J.CECA.2016.01.008
Steinman L, Altmann D, Sansom D et al (1996) Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85:299–302. https://doi.org/10.1016/S0092-8674(00)81107-1
Lee SY, Giladi M, Bohbot H et al (2016) Structure-dynamic basis of splicing-dependent regulation in tissue-specific variants of the sodium-calcium exchanger. FASEB J 30:1356–1366. https://doi.org/10.1096/fj.15-282251
Giladi M, Bohbot H, Buki T et al (2012) Dynamic features of allosteric Ca2+ sensor in tissue-specific NCX variants. Cell Calcium 51:478–485. https://doi.org/10.1016/j.ceca.2012.04.007
Matsuoka S, Nicoll DA, He Z, Philipson KD (1997) Regulation of cardiac Na(+)-Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109:273–286. https://doi.org/10.1085/jgp.109.2.273
Li Z, Nicoll DA, Collins A et al (1991) Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)-Ca2+ exchanger. J Biol Chem 266:1014–1020
Giladi M, Tal I, Khananshvili D (2016) Structural Features of Ion Transport and Allosteric Regulation in Sodium-Calcium Exchanger (NCX) Proteins. Front Physiol 7:30. https://doi.org/10.3389/fphys.2016.00030
Hilgemann DW, Collins A, Matsuoka S (1992) Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol 100:933–961. https://doi.org/10.1085/jgp.100.6.933
Chernysh O, Condrescu M, Reeves JP (2008) Sodium-dependent inactivation of sodium/calcium exchange in transfected Chinese hamster ovary cells. Am J Physiol Cell Physiol 295:C872–C882. https://doi.org/10.1152/ajpcell.00221.2008
Verkhratsky A, Trebak M, Perocchi F et al (2018) Crosslink between calcium and sodium signalling. Exp Physiol 103:157–169. https://doi.org/10.1113/EP086534
Michel LYM, Verkaart S, Koopman WJH et al (2014) Function and regulation of the Na+–Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. J Biol Chem 289:11293–11303. https://doi.org/10.1074/jbc.M113.529388
DiPolo R, Beaugé L (2006) Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol Rev 86:155–203
Berberián G, Bollo M, Montich G et al (2009) A novel lipid binding protein is a factor required for MgATP stimulation of the squid nerve Na+/Ca2+ exchanger. Biochim Biophys Acta 1788:1255–1262. https://doi.org/10.1016/j.bbamem.2008.12.016
Cousido-Siah A, Ayoub D, Berberián G et al (2012) Structural and functional studies of ReP1-NCXSQ, a protein regulating the squid nerve Na+/Ca2+ exchanger. Acta Crystallogr D 68:1098–1107. https://doi.org/10.1107/S090744491202094X
Lariccia V, Amoroso S (2018) Calcium- and ATP-dependent regulation of Na/Ca exchange function in BHK cells: comparison of NCX1 and NCX3 exchangers. Cell Calcium 73:95–103. https://doi.org/10.1016/j.ceca.2018.04.007
Dyck C, Omelchenko A, Elias CL et al (1999) Ionic regulatory properties of brain and kidney splice variants of the NCX1 Na-Ca 2 exchanger. J Gen Physiol 114:701–711. https://doi.org/10.1085/jgp.114.5.701
Matsuoka S (2004) Forefront of Na+/Ca2+ exchanger studies: regulation kinetics of Na+/Ca2+ exchangers. J Pharmacol Sci 96:12–14. https://doi.org/10.1254/jphs.fmj04002x2
Quednau BD, Nicoll D, Philipson KD (1997) Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCXl, NCX2, and NCX3 in rat. Am J Physiol 272:C1250–C1261. https://doi.org/10.1152/ajpcell.1997.272.4.C1250
Nakasaki Y, Iwamoto T, Hanada H et al (1993) Cloning of the rat aortic smooth muscle Na +/Ca2 + exchanger and tissue-specific expression of isoforms1. J Biochem 114:528–534. https://doi.org/10.1093/oxfordjournals.jbchem.a124211
Rose CR, Ransom BR (1996) Mechanisms of H+ and Na+ changes induced by glutamate, kainate, and D-aspartate in rat hippocampal astrocytes. J Neurosci 16:5393–5404
Nicholas SB, Yang W, Lee SL et al (1998) Alternative promoters and cardiac muscle cell-specific expression of the Na+/Ca2+ exchanger gene. Am J Physiol 274:H217–H232. https://doi.org/10.1152/ajpheart.1998.274.1.H217
Barnes KV, Cheng G, Dawson MM, Menick DR (1997) Cloning of cardiac, kidney, and brain promoters of the feline ncx1 gene. J Biol Chem 272:11510–11517
Koban MU, Brugh SA, Riordon DR et al (2001) A distant upstream region of the rat multipartite Na(+)-Ca(2+) exchanger NCX1 gene promoter is sufficient to confer cardiac-specific expression. Mech Dev 109:267–279. https://doi.org/10.1016/s0925-4773(01)00548-2
Lee S-L, Yu ASL, Lytton J (1994) Tissue-specific Expression of Na + -Ca2 + exchanger Isoforms. J Biol Chem 269:14849–14852
Yu X-M, Groveman BR, Fang X-Q, Lin S-X (2010) The role of intracellular sodium (Na+) in the regulation of calcium (Ca2+)-mediated signaling and toxicity. Health (Irvine Calif) 02:8–15. https://doi.org/10.4236/health.2010.21002
Stadelmann C, Timmler S, Barrantes-Freer A, Simons M (2019) Myelin in the Central Nervous System: structure, Function, and Pathology. Physiol Rev 99:1381–1431. https://doi.org/10.1152/physrev.00031.2018
Pfeiffer S, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3:191–197. https://doi.org/10.1016/0962-8924(93)90213-K
Michalski JP, Kothary R (2015) Oligodendrocytes in a nutshell. Front Cell Neurosci 9:340. https://doi.org/10.3389/fncel.2015.00340
Elbaz B, Popko B (2019) Molecular control of oligodendrocyte development. Trends Neurosci 42:263–277. https://doi.org/10.1016/j.tins.2019.01.002
Tiane A, Schepers M, Rombaut B et al (2019) From OPC to oligodendrocyte: an epigenetic journey. Cells. https://doi.org/10.3390/cells8101236
Sock E, Wegner M (2019) Transcriptional control of myelination and remyelination. Glia 67:2153–2165. https://doi.org/10.1002/glia.23636
Wheeler NA, Fuss B (2016) Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp Neurol 283:512–530. https://doi.org/10.1016/j.expneurol.2016.03.019
Yu L, Colvin RA (1997) Regional differences in expression of transcripts for Na+/Ca2+ exchanger isoforms in rat brain. Mol Brain Res 50:285–292. https://doi.org/10.1016/S0169-328X(97)00202-7
Tong XP, Li XY, Zhou B et al (2009) Ca2 + signaling evoked by activation of Na + channels and Na +/Ca2 + exchangers is required for GABA-induced NG2 cell migration. J Cell Biol 186:113–128. https://doi.org/10.1083/jcb.200811071
Steffensen I, Waxman SG, Mills L, Stys PK (1997) Immunolocalization of the Na+-Ca2+ exchanger in mammalian myelinated axons. Brain Res 776:1–9. https://doi.org/10.1016/S0006-8993(97)00868-8
Boscia F, D’avanzo C, Pannaccione A et al (2012) Silencing or knocking out the Na +/Ca2 + exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ 19:562–572. https://doi.org/10.1038/cdd.2011.125
Boscia F, D’Avanzo C, Pannaccione A et al (2013) New roles of NCX in glial cells: activation of microglia in ischemia and differentiation of oligodendrocytes. Adv Exp Med Biol 961:307–316. https://doi.org/10.1007/978-1-4614-4756-6_26
Simpson PB, Armstrong RC (1999) Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia 26:22–35
Paez PM, Fulton D, Colwell CS, Campagnoni AT (2009) Voltage-operated Ca2+ and Na+ channels in the oligodendrocyte lineage. J Neurosci Res 87:3259–3266. https://doi.org/10.1002/jnr.21938
Habermacher C, Angulo MC, Benamer N (2019) Glutamate versus GABA in neuron-oligodendroglia communication. Glia 67:2092–2106. https://doi.org/10.1002/glia.23618
Friess M, Hammann J, Unichenko P et al (2016) Intracellular ion signaling influences myelin basic protein synthesis in oligodendrocyte precursor cells. Cell Calcium 60:322–330. https://doi.org/10.1016/j.ceca.2016.06.009
Hammann J, Bassetti D, White R et al (2018) α2 isoform of Na+, K+-ATPase via Na+, Ca2+ exchanger modulates myelin basic protein synthesis in oligodendrocyte lineage cells in vitro. Cell Calcium 73:1–10. https://doi.org/10.1016/j.ceca.2018.03.003
Thakurela S, Garding A, Jung RB et al (2016) The transcriptome of mouse central nervous system myelin. Sci Rep 6:25828. https://doi.org/10.1038/srep25828
Martinez-Lozada Z, Waggener CT, Kim K et al (2014) Activation of sodium-dependent glutamate transporters regulates the morphological aspects of oligodendrocyte maturation via signaling through calcium/calmodulin-dependent kinase IIβ’s actin-binding/-stabilizing domain. Glia 62:1543–1558. https://doi.org/10.1002/glia.22699
Suárez-Pozos E, Thomason EJ, Fuss B (2019) Glutamate transporters: expression and function in oligodendrocytes. Neurochem Res. https://doi.org/10.1007/s11064-018-02708-x
Martínez-Lozada Z, Hernández-Kelly LC, Aguilera J et al (2011) Signaling through EAAT-1/GLAST in cultured Bergmann glia cells. Neurochem Int 59:871–879. https://doi.org/10.1016/j.neuint.2011.07.015
Iwamoto T, Kita S, Shigekawa M (2002) Functional analysis of Na+/Ca2+ exchanger using novel drugs and genetically engineered mice. Nihon Yakurigaku Zasshi 120:91P–93P
Iwamoto T, Inoue Y, Ito K et al (2004) The exchanger inhibitory peptide region-dependent inhibition of Na+/Ca2+ exchange by SN-6 [2-[4-(4-nitrobenzyloxy)benzyl]- thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol Pharmacol 66:45–55. https://doi.org/10.1124/mol.66.1.45
Gong XQ, Frandsen A, Lu WY et al (2005) D-aspartate and NMDA, but not L-aspartate, block AMPA receptors in rat hippocampal neurons. Br J Pharmacol 145:449–459. https://doi.org/10.1038/sj.bjp.0706199
Sugiyama H, Ito I, Watanabe M (1989) Glutamate receptor subtypes may be classified into two major categories: a study on Xenopus oocytes injected with rat brain mRNA. Neuron 3:129–132. https://doi.org/10.1016/0896-6273(89)90121-9
Bender AS, Woodbury DM, White HS (1997) The rapid L- and D-aspartate uptake in cultured astrocytes. Neurochem Res 22:721–726. https://doi.org/10.1023/a:1027358211472
de Rosa V, Secondo A, Pannaccione A et al (2019) d-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol Med 11:e9278. https://doi.org/10.15252/emmm.201809278
Kukley M, Capetillo-Zarate E, Dietrich D (2007) Vesicular glutamate release from axons in white matter. Nat Neurosci 10:311–320. https://doi.org/10.1038/nn1850
Ziskin JL, Nishiyama A, Rubio M et al (2007) Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 10:321–330. https://doi.org/10.1038/nn1854
Wake H, Lee PR, Fields RD (2011) Control of local protein synthesis…background. Science 333:1647–1652. https://doi.org/10.1126/science.1206998
Foster AY, Bujalka H, Emery B (2019) Axoglial interactions in myelin plasticity: evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia 67:2038–2049. https://doi.org/10.1002/glia.23629
Monje M (2018) Myelin plasticity and nervous system function. Annu Rev Neurosci 41:61–76. https://doi.org/10.1146/annurev-neuro-080317-061853
Chorghay Z, Káradóttir RT, Ruthazer ES (2018) White matter plasticity keeps the brain in tune: axons conduct while glia wrap. Front Cell Neurosci 12:428. https://doi.org/10.3389/fncel.2018.00428
Bechler ME, Swire M, Ffrench-Constant C (2018) Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Dev Neurobiol 78:68–79. https://doi.org/10.1002/dneu.22518
Back SA, Luo NL, Borenstein NS et al (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302–1312. https://doi.org/10.1523/jneurosci.21-04-01302.2001
Cai Q, Ma T, Tian Y et al (2018) Catalpol inhibits ischemia-induced premyelinating oligodendrocyte damage through regulation of intercellular calcium homeostasis via Na+/Ca2+ exchanger 3. Int J Mol Sci. https://doi.org/10.3390/ijms19071925
Ma T, Wu X, Cai Q et al (2015) Lead poisoning disturbs oligodendrocytes differentiation involved in decreased expression of NCX3 inducing intracellular calcium overload. Int J Mol Sci 16:19096–19110. https://doi.org/10.3390/ijms160819096
Deng W, McKinnon RD, Poretz RD (2001) Lead exposure delays the differentiation of oligodendroglial progenitors in vitro. Toxicol Appl Pharmacol 174:235–244. https://doi.org/10.1006/TAAP.2001.9219
Pignataro G, Cuomo O, Vinciguerra A et al (2013) NCX as a key player in the neuroprotection exerted by ischemic preconditioning and postconditioning. Adv Exp Med Miol 961:223–240. https://doi.org/10.1007/978-1-4614-4756-6_19
Pignataro G, Gala R, Cuomo O et al (2004) Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke 35:2566–2570. https://doi.org/10.1161/01.STR.0000143730.29964.93
Gerkau NJ, Rakers C, Durry S et al (2018) Reverse NCX attenuates cellular sodium loading in metabolically compromised cortex. Cereb Cortex 28:4264–4280. https://doi.org/10.1093/cercor/bhx280
Chen H, Kintner DB, Jones M et al (2007) AMPA-mediated excitotoxicity in oligodendrocytes: role for Na+-K+-Cl—co-transport and reversal of Na+/Ca2+ exchanger. J Neurochem 102:1783–1795. https://doi.org/10.1111/j.1471-4159.2007.04638.x
Stover JF, Pleines UE, Morganti-Kossmann MC et al (1997) Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur J Clin Invest 27:1038–1043. https://doi.org/10.1046/j.1365-2362.1997.2250774.x
Macrez R, Stys PK, Vivien D et al (2016) Mechanisms of glutamate toxicity in multiple sclerosis: biomarker and therapeutic opportunities. Lancet Neurol 15:1089–1102. https://doi.org/10.1016/S1474-4422(16)30165-X
Newcombe J, Uddin A, Dove R et al (2008) Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol 18:52–61. https://doi.org/10.1111/j.1750-3639.2007.00101.x
Follett PL, Deng W, Dai W et al (2004) Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci 24:4412–4420. https://doi.org/10.1523/JNEUROSCI.0477-04.2004
McCracken E, Fowler JH, Dewar D et al (2002) Grey matter and white matter ischemic damage is reduced by the competitive AMPA receptor antagonist, SPD 502. J Cereb Blood Flow Metab 22:1090–1097. https://doi.org/10.1097/00004647-200209000-00006
Park E, Velumian AA, Fehlings MG (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21:754–774. https://doi.org/10.1089/0897715041269641
Rosenberg LJ, Teng YD, Wrathall JR (1999) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci 19:464–475
Wosik K, Ruffini F, Almazan G et al (2004) Resistance of human adult oligodendrocytes to AMPA/kainate receptor-mediated glutamate injury. Brain 127:2636–2648. https://doi.org/10.1093/brain/awh302
Itoh T, Beesley J, Itoh A et al (2002) AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem 81:390–402. https://doi.org/10.1046/j.1471-4159.2002.00866.x
Ceprian M, Fulton D (2019) Glial cell AMPA receptors in nervous system health, injury and disease. Int J Mol Sci. https://doi.org/10.3390/ijms20102450
Fan X, Xiong Y, Wang Y (2019) A reignited debate over the cell(s) of origin for glioblastoma and its clinical implications. Front Med 13:531–539. https://doi.org/10.1007/s11684-019-0700-1
Hsu LS, Chou WY, Chueh SH (1995) Evidence for a Na+/Ca2+ exchanger in neuroblastoma x glioma hybrid NG108-15 cells. Biochem J 309:445–452. https://doi.org/10.1042/bj3090445
Amoroso S, De Maio M, Russo GM et al (1997) Pharmacological evidence that the activation of the Na(+)-Ca2+ exchanger protects C6 glioma cells during chemical hypoxia. Br J Pharmacol 121:303–309. https://doi.org/10.1038/sj.bjp.0701092
Rodrigues T, Estevez GNN, Tersariol ILDS (2019) Na +/Ca2 + exchangers: unexploited opportunities for cancer therapy? Biochem Pharmacol 163:357–361. https://doi.org/10.1016/j.bcp.2019.02.032
Hu H-J, Wang S-S, Wang Y-X et al (2019) Blockade of the forward Na+/Ca2+ exchanger suppresses the growth of glioblastoma cells through Ca2+-mediated cell death. Br J Pharmacol 176:2691–2707. https://doi.org/10.1111/bph.14692
Marques S, Zeisel A, Codeluppi S et al (2016) Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352:1326–1329. https://doi.org/10.1126/science.aaf6463
Foerster S, Hill MFE, Franklin RJM (2019) Diversity in the oligodendrocyte lineage: plasticity or heterogeneity? Glia 67:1797–1805. https://doi.org/10.1002/glia.23607
Spitzer SO, Sitnikov S, Kamen Y et al (2019) Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 101:459.e5–471.e5. https://doi.org/10.1016/j.neuron.2018.12.020
Jäkel S, Agirre E, Mendanha Falcão A et al (2019) Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566:543–547. https://doi.org/10.1038/s41586-019-0903-2
Watanabe Y (2019) Cardiac Na+/Ca2+ exchange stimulators among cardioprotective drugs. J Physiol Sci. https://doi.org/10.1007/s12576-019-00721-5
Song S, Luo L, Sun B, Sun D (2019) Roles of glial ion transporters in brain diseases. Glia. https://doi.org/10.1002/glia.23699
Acknowledgements
The authors are supported by Grants from the National Institute of Health (Grant No.: R01NS045883; BF), the National Multiple Sclerosis Society (Grant No.: RG-1506-04546; BF) and the Commonwealth Health Research Board (Grant No.: 236-04-17 BF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In honor of Professor Michael Robinson.
Rights and permissions
About this article
Cite this article
Spencer, S.A., Suárez-Pozos, E., Escalante, M. et al. Sodium–Calcium Exchangers of the SLC8 Family in Oligodendrocytes: Functional Properties in Health and Disease. Neurochem Res 45, 1287–1297 (2020). https://doi.org/10.1007/s11064-019-02949-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02949-4