Abstract
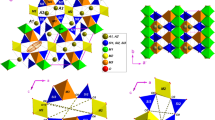
To explain the anomalous anisotropy in thermal expansion properties reported in rhodochrosite (MnCO3) previously Rao and Murthy (J Mater Sci 5: 82, 1970), Li et al. (High Temp High Press, 2019), the evaluation of crystal structure is thought to be indispensable as an important aspect in mineralogy. In this spirit, single crystals of impurity-free rhodochrosite, up to 100 μm in size, were synthesized under high-pressure–temperature (P–T) conditions. The standard crystal structure, without the impurities common to natural samples, was investigated by means of single-crystal X-ray diffraction (XRD). The unit cell parameters obtained for the \(R\overline{3}c\) symmetry were a = 4.7754(5) Å and c = 15.6484(18) Å, with a final R value of 0.0162. The (MnO6) octahedron exhibits an anomalous bond angle that tends more toward 90° of a regular octahedron, which is totally different from those of MgCO3, FeCO3, and CaCO3. Using the single-crystal XRD from 100 to 370 K, the thermal expansion coefficients were quantified as αa = 5.08 × 10−6 K−1 and αc = 18.06 × 10–6 K−1, as well as αVunit cell = 28.49 × 10–6 K−1. The geometry of (MnO6) octahedron as function of temperature was also determined as αMn–O = 12.14 × 10−6 K−1 and αO–Mn–O ≈ 0.05°/100 K. The anisotropy of MnCO3 (αa/αc = 3.55), similar to that of MgCO3 (~ 3.0, Markgraf and Reeder, Am Mineral, 70: 590–600, 1985), indicates that the difference in bond angle has no significant effect on the thermal expansion properties. According to the standard crystal structures of end members (MgCO3, FeCO3, MnCO3, and CaCO3), the cation substitution in calcite-type structures is proven to agree with the rigid body model and the linear solid solution relationship is highly consistent with those of natural carbonates.





Similar content being viewed by others
References
Biellmann C, Gillet P, Guyot FO, Peyronneau J, Reynard B (1993) Experimental evidence for carbonate stability in the Earth’s lower mantle. Earth Planet Sci Lett 118:31–41
Burke IT, Kemp AES (2002) Microfabric analysis of Mn-carbonate laminae deposition and Mn-sulfide formation in the Gotland Deep, Baltic Sea. Geochim Cosmochim Acta 66:1589–1600
Cerantola V, McCammon C, Kupenko I, Kantor I, Marini C, Wilke M, Ismailova L, Solopova N, Chumakov A, Pascarelli S, Dubrovinsky L (2015) High-pressure spectroscopic study of siderite (FeCO3) with a focus on spin rossover. Am Mineral 100:2670–2681
Cerantola V, Bykova E, Kupenko I, Merlini M, Ismailova L, McCammon C, Bykov M, Chumakov AI, Petitgirard S, Kantor I, Svitlyk V, Jacobs J, Hanfland M, Mezouar M, Prescher C, Ruffer R, Prakapenka VB, Dubrovinsky L (2017) Stability of iron-bearing carbonates in the deep Earth’s interior. Nat Commun 8:15960
Dasgupta R, Hirschmann MM (2010) The deep carbon cycle and melting in Earth’s interior. Earth Planet Sci Lett 298:1–13
Duan J, Fu Y, Zhang Z, Ma X, Xiao J (2019) The metallogenic environment of the Dounan manganese deposit, Southeast Yunnan, China: evidence from geochemistry and Moesbauer spectroscopic. Acta Geochim 38:78–94
Effenberger H, Mereiter K, Zemann J (1981) Crystal structure refinements of magnesite, calcite, rhodochrosite, siderite, smithonite, and dolomite, with discussion of some aspects of the stereochemistry of calcite type carbonates. Zeitschrift fur Kristallographie 156:233–243
Fan D, Hein JR, Ye J (1999) Ordovician reef-hosted Jiaodingshan Mn–Co deposit and Dawashan Mn deposit, Sichuan Province, China. Ore Geol Rev 15:135–151
Farfan GA, Boulard E, Wang S, Mao WL (2013) Bonding and electronic changes in rhodochrosite at high pressure. Am Mineral 98:1817–1823
Fiquet G, Guyot F, Itie LP (1994) High-pressure X-ray diffraction study of carbonates: MgCO3, CaMg(CO3)2, and CaCO3. Am Mineral 79:15–23
Gillet P, Biellmann C, Reynard B, McMillan P (1993) Raman spectroscopic studies of carbonates part I: high-pressure and high-temperature behaviour of calcite, magnesite, dolomite and aragonite. Phys Chem Miner 20:1–18
Graf DL (1961) Crystallographic tables for the rhombohedral carbonates. Am Mineral 46:1283–1316
Hazen RM, Finger L (1982) Comparative crystal chemistry: temperature, pressure, composition and the variation of crystal structure. Wiley, New York, p 228
Hazen RM, Hemley RJ, Mangum AJ (2012) Carbon in Earth's interior: storage, cycling, and life. Eos Trans Am Geophys Union 93(2):17–18
Ishizawa N, Setoguchi H, Yanagisawa K (2013) Structural evolution of calcite at high temperatures: phase V unveiled. Sci Rep 3:2832
Isshiki M, Irifune T, Hirose K, Ono S, Ohishi Y, Watanuki T, Nishibori E, Takata M, Sakata M (2004) Stability of magnesite and its high-pressure form in the lowermost mantle. Nature 427:60–63
Javoy M (1997) The major volatile elements of the Earth: their origin, behaviour, and fate. Geophys Res Lett 24:177–180
Lavina B, Dera P, Downs RT, Prakapenka V, Rivers M, Sutton S, Nicol M (2009) Siderite at lower mantle conditions and the effects of the pressure-induced spin-pairing transition. Geophys Res Lett 36:L23306
Li R, Liang W, He H, Meng Y, Tang H (2019) The high-pressure synthesis and thermal expansivity investigation of carbonates solid solutions Mg1–xMnxCO3. High Temp High Press 48:367–380
Liang W, Li Z, Yin Y, Li R, Chen L, He Y, Dong H, Dai L, Li H (2018a) Single crystal growth, characterization and high-pressure Raman spectroscopy of impurity-free magnesite (MgCO3). Phys Chem Miner 45:423–434
Liang W, Yin Y, Li Z, Li R, Lin L, He Y, Dong H, Li Z, Yan S, Zhai S, Li H (2018b) Single crystal growth, crystalline structure investigation and high-pressure behavior of impurity-free siderite (FeCO3). Phys Chem Miner 45:831–842
Liang W, Chen L, Wang L, Yin Y, Li Z, Li H (2018c) High pressure synthesis of siderite (FeCO3) and its thermal expansion coefficient. High Temp High Press 47:153–164
Lin J-F, Liu J, Jacobs C, Prakapenka VB (2012) Vibrational and elastic properties of ferromagnesite across the electronic spin-pairing transition of iron. Am Mineral 97:583–591
Litasov KD, Ohtani E (2009) Solidus and phase relations of carbonated peridotite in the system CaO–Al2O3–MgO–SiO2–Na2O–CO2 to the lower mantle depths. Phys Earth Planet Inter 177:46–58
Liu L-G, Lin C-C, Yang Y-J (2001) Formation of diamond by decarbonation of MnCO3. Solid State Commun 118:195–198
Liu J, Lin J-F, Mao Z, Prakapenka VB (2014) Thermal equation of state and spin transition of magnesiosiderite at high pressure and temperature. Am Mineral 99:84–93
Liu J, Caracas R, Fan D, Bobocioiu E, Zhang D, Mao WL (2016) High-pressure compressibility and vibrational properties of (Ca, Mn)CO3. Am Mineral 101:2723–2730
Ma H, Xu Y, Huang K, Sun Y, Ke S, Peng Y, Shen B (2018) Heterogeneous Mg isotopic composition of the early Carboniferous limestone: implications for carbonate as a seawater archive. Acta Geochim 37:1–18
Markgraf SA, Reeder RJ (1985) High-temperature structure refinements of calcite and magnesite. Am Mineral 70:590–600
Martens R, Rosenhauer M, Gehlen KV (1982) Compressibilities of carbonates. In: Schreyer W (ed) High-pressure researches in geoscience. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, pp 215–222
Maslen EN, Streltsov VA, Streltsova NR (1995) Electron density and optical anisotropy in rhombohedral carbonates. III. Synchrotron X-ray studies of CaCO3, MgCO3 and MnCO3. Acta Crystallogr B51:929–939
Megaw HD (1971) Crystal structures and thermal expansion. Mater Res Bull 6:1007–1018
Merlini M, Hanfland M, Gemmi M (2015) The MnCO3-II high-pressure polymorph of rhodochrosite. Am Mineral 100:2625–2629
Merlini M, Sapelli F, Fumagalli P, Gatta GD, Lotti P, Tumiati S, Aabdellatief M, Lausi A, Plaisier J, Hanfland M, Crichton W, Chantel J, Guignard J, Meneghini C, Pavese A, Poli P (2016) High-temperature and high-pressure behavior of carbonates in the ternary diagram CaCO3–MgCO3–FeCO3. Am Mineral 101:1423–1430
Oelkers EH, Cole DR (2008) Carbon dioxide sequestration a solution to a global problem. Elements 4:305–310
Ono S, Kikegawa T, Ohishi Y (2007) High-pressure transition of CaCO3. Am Mineral 92:1246–1249
Ozturk H, Frakes LA (1995) Sedimentation and diagenesis of an Oligocene manganese deposit in a shallow sub-basin of the Paratethys: Thrace Basin, Turkey. Ore Geol Rev 10:117–132
Peacor DR, Essene EJ, Gaines AM (1987) Petrologic and crystal-chemical implications of cation order-disorder in kutnahorite CaMn(CO3)2. Am Mineral 72:319–328
Rao KVK, Murthy KS (1970) Thermal expansion of manganese carbonate. J Mater Sci 5:82–83
Reeder RJ (1983) Crystal chemistry of the rhombohedral carbonates. In: Reeder RJ (ed) Carbonates: mineralogy and chemistry. Reviews in mineralogy, 11. Mineralogical Society of America, Washington, DC, pp 1–48
Reeder RJ, Dollase WA (1989) Structural variation in the dolomite-ankerite solid-solution series: an X-ray, Moessbauer, and TEM study. Am Mineral 74:1159–1167
Rosenberg PE (1963) Synthetic solid solutions in the systems MgCO3–FeCO3 and MnCO3–FeCO3. Am Mineral 48:1396–1400
Ross NL (1997) The equation of state and high-pressure behavior of magnesite. Am Mineral 82:682–688
Santillan J, Williams Q (2004) A high-pressure infrared and X-ray study of FeCO3 and MnCO3: comparison with CaMg(CO3)2-dolomite. Phys Earth Planet Inter 143–144:291–304
Seto Y, Hamane D, Nagai T, Fujino F (2008) Fate of carbonates within oceanic plates subducted to the lower mantle, and a possible mechanism of diamond formation. Phys Chem Miner 35:223–229
Shannon RD, Prewitt CT (1969) Effective ionic radii in oxides and fluorides. Acta Crystallogr A B25:925–946
Sheldrick GM (2008) Acta Crystallogr A 2008(64):112–122
Spivak A, Solopova N, Cerantola V, Bykova E, Zakharchenko E, Dubrovinsky L, Litvin Y (2014) Raman study of MgCO3–FeCO3 carbonate solid solution at high pressures up to 55 GPa. Phys Chem Miner 41:633–638
Suito K, Namba J, Horikawa T, Taniguchi Y, Sakurai N, Kobayashi M, Onodera A, Shimomura O, Kikegawa T (2001) Phase relations of CaCO3 at high pressure and high temperature. Am Mineral 86:997–1002
Williams Q, Collerson B, Knittle E (1992) Vibrational spectra of magnesite (MgCO3) and calcite-III at high pressures. Am Mineral 77:1158–1165
Zhang J, Martinez I, Guyot F, Gillet P, Saxena SK (1997) X-ray diffraction study of magnesite at high pressure and high temperature. Phys Chem Miner 24:122–130
Acknowledgements
We appreciate two anonymous reviewers for their valuable comments and suggestions. We acknowledge Jung-Fu Lin from University of Texas at Austin for constructive discussion in carbonate minerals. This work was financially supported by Major State Research Development Program of China (2016YFC0601101), the National Science Foundation for Young Scientists of China (41802044), National Natural Fund of China (4160030283), the Strategic Priority Research Program (B) of Chinese Academy of Sciences (XDB 18010401), 135 Program of the Institute of Geochemistry (Y2ZZ041000), CAS, and the Western Light (Y8CR028).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, W., Li, L., Li, R. et al. Crystal structure of impurity-free rhodochrosite (MnCO3) and thermal expansion properties. Phys Chem Minerals 47, 9 (2020). https://doi.org/10.1007/s00269-019-01078-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00269-019-01078-2