Abstract
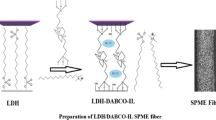
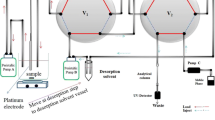
A CuCr-layered double hydroxide nanosheet intercalated with terephthalic acid (TPA/LDH) was introduced as a coating for the in-tube solid phase microextraction (IT-SPME). The coating was placed on the inner surface of a stainless steel tube by using two-electrode electrodeposition. The sorbent was characterized by X-ray diffraction, scanning electronic microscopy, and Fourier transform infrared spectroscopy. The TPA/LDH coating, compared to a nitrate-LDH coating, exhibits enhanced extraction efficiency, long lifetime, good mechanical stability, and a large specific surface. The method was used for the extraction, preconcentration, and subsequent HPLC-based determination of dimethyl phthalate (DMP), dibutyl phthalate (DBP), diallyl phthalate (DAP), and diethylhexyl phthalate (DEHP). The effects of pH value of the solution, salt concentration, extraction and desorption conditions, and the effect of the alcohol content of the solution on the extraction efficiency were optimized. Under optimal conditions, the response is linear in the 0.05 to 1000 μg L−1 ester concentration range, and the limits of detection (at S/N = 3) range between 0.01 to 0.1 μg L−1. The inter- and intra-assay precisions (RSD%, for n = 3) range from 3.8 to 6.8% and from 3.5 to 5.7%, respectively. The method was successfully applied to the determination of four phthalate esters in different beverage samples.

A CuCr-layered double hydroxide nanosheet intercalated with terephthalic acid was used as a coating for in-tube solid phase microextraction of some phthalate esters from beverage samples.



Similar content being viewed by others
References
Pawliszyn J, Arthur CL (1990) Solid phase Microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62:2145–2148. https://doi.org/10.1021/ac00218a019
Lashgari M, Yamini Y (2019) An overview of the most common lab-made coating materials in solid phase microextraction. Talanta 191:283–306. https://doi.org/10.1016/j.talanta.2018.08.077
Kataoka H, Ishizaki A, Nonaka Y, Saito K (2009) Developments and applications of capillary microextraction techniques: a review. Anal Chim Acta 655:8–29. https://doi.org/10.1016/j.aca.2009.09.032
Taylor P, Eisert R, Pawliszyn J (2006) New trends in solid-phase microextraction. Crit Rev Anal Chem 27:103–135. https://doi.org/10.1080/10408349708050585
Wang X, Huang P, Ma X, Du X, Lu X (2019) Enhanced in-out-tube solid-phase microextraction by molecularly imprinted polymers-coated capillary followed by HPLC for endocrine disrupting chemicals analysis. Talanta 194:7–13. https://doi.org/10.1016/j.talanta.2018.10.027
Ho TD, Canestraro AJ, Anderson JL (2011) Ionic liquids in solid-phase microextraction : a review. Anal Chim Acta 695:18–43. https://doi.org/10.1016/j.aca.2011.03.034
Piri-Moghadam H, Alam MN, Pawliszyn J (2017) Review of geometries and coating materials in solid phase microextraction: opportunities, limitations, and future perspectives. Anal Chim Acta 984:42–65. https://doi.org/10.1016/j.aca.2017.05.035
Shamsayei M, Yamini Y, Asiabi H, Safari M (2018) On-line packed magnetic in-tube solid phase microextraction of acidic drugs such as naproxen and indomethacin by using Fe3O4@SiO2@layered double hydroxide nanoparticles with high anion exchange capacity. Microchim Acta 185:192. https://doi.org/10.1007/s00604-018-2716-7
Sansuk S, Nanan S, Srijaranai S (2015) New eco-friendly extraction of anionic analytes based on formation of layered double hydroxides. Green Chem 17:3837–3843. https://doi.org/10.1039/c5gc00713e
Asiabi H, Yamini Y, Shamsayei M (2017) Highly selective and efficient removal of arsenic(V), chromium(VI) and selenium(VI) oxyanions by layered double hydroxide intercalated with zwitterionic glycine. J Hazard Mater 339:239–247. https://doi.org/10.1016/j.jhazmat.2017.06.042
Yu S, Wang X, Chen Z, Wang J, Wang S, Hayat T, Wang X (2017) Layered double hydroxide intercalated with aromatic acid anions for the efficient capture of aniline from aqueous solution. J Hazard Mater 321:111–120. https://doi.org/10.1016/j.jhazmat.2016.09.009
Yu Q, Zheng Y, Wang Y, Shen L, Wang H, Zheng Y, He N, Li Q (2015) Highly selective adsorption of phosphate by pyromellitic acid intercalated ZnAl-LDHs : assembling hydrogen bond acceptor sites. Chem Eng J 260:809–817. https://doi.org/10.1016/j.cej.2014.09.059
Mishra G, Dash B, Pandey S (2018) Applied clay science layered double hydroxides : a brief review from fundamentals to application as evolving biomaterials. Appl Clay Sci 153:172–186. https://doi.org/10.1016/j.clay.2017.12.021
Zhang F, Hou W (2018) Mechano-hydrothermal preparation of Li-Al-OH layered double hydroxides. Solid State Sci 79:93–98. https://doi.org/10.1016/j.solidstatesciences.2018.03.007
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) Nanosheets. Chem Rev 112:4124–4155. https://doi.org/10.1021/cr200434v
Lu Z, Xu W, Zhu W, Yang Q, Lei X, Liu J, Li Y, Sun X, Duan X (2014) Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem Commun 50:6479–6482. https://doi.org/10.1039/c4cc01625d
Soltani R, Shahvar A, Dinari M, Saraji M (2017) Environmentally-friendly and ultrasonic-assisted preparation of two-dimensional ultrathin Ni/Co-NO3 layered double hydroxide nanosheet for micro solid-phase extraction of phenolic acids from fruit juices. Ultrason Sonochem 40:395–401. https://doi.org/10.1016/j.ultsonch.2017.07.031
Sheng L, Liu J, Zhang C, Zou L, Li Y, Xu ZP (2018) Pretreating anaerobic fermentation liquid with calcium addition to improve short chain fatty acids extraction via in situ synthesis of layered double hydroxides. Bioresour Technol 271:190–195. https://doi.org/10.1016/j.biortech.2018.09.086
Asiabi H, Yamini Y, Shamsayei M (2018) Using cobalt/chromium layered double hydroxide nano-sheets as a novel packed in-tube solid phase microextraction sorbent for facile extraction of acidic pesticides from water samples. New J Chem 42:9935–9944. https://doi.org/10.1039/C8NJ00372F
Wang FQ, Li J, Wu JF, Zhao GC (2018) Layered double hydroxides as a coating for the determination of phthalate esters in aqueous solution with solid-phase microextraction followed by gas chromatography. Chromatographia 81:799–807. https://doi.org/10.1007/s10337-018-3507-3
Wu Q, Liu M, Ma X, Wang W (2012) Extraction of phthalate esters from water and beverages using a graphene-based magnetic nanocomposite prior to their determination by HPLC. Microchim Acta 177:23–30. https://doi.org/10.1007/s00604-011-0752-7
Gualandi I, Monti M, Scavetta E, Tonelli D, Prevot V, Mousty C (2015) Electrodeposition of layered double hydroxides on platinum : insights into the reactions sequence. Electrochim Acta 152:75–83. https://doi.org/10.1016/j.electacta.2014.11.096
Forticaux A, Dang L, Liang H, Jin S (2015) Controlled synthesis of layered double hydroxide controlled synthesis of layered double hydroxide nanoplates driven by screw dislocations. Nano Lett 15:3403–3409. https://doi.org/10.1021/acs.nanolett.5b00758
Shamsayei M, Yamini Y, Asiabi H (2018) Electrochemically controlled fiber-in-tube solid-phase microextraction method for the determination of trace amounts of antipsychotic drugs in biological samples. J Sep Sci 41:3598–3606. https://doi.org/10.1002/jssc.201800417
Lashgari M, Yamini Y (2018) Fiber-in-tube solid-phase microextraction of caffeine as a molecular tracer in wastewater by electrochemically deposited layered double hydroxide. J Sep Sci 41:2393–2400. https://doi.org/10.1002/jssc.201701539
Cinelli G, Avino P, Notardonato I, Centola A, Russo MV (2013) Rapid analysis of six phthalate esters in wine by ultrasound-vortex-assisted dispersive liquid-liquid micro-extraction coupled with gas chromatography-flame ionization detector or gas chromatography-ion trap mass spectrometry. Anal Chim Acta 769:72–78. https://doi.org/10.1016/j.aca.2013.01.031
Fan Y, Liu S, Xie Q (2014) Rapid determination of phthalate esters in alcoholic beverages by conventional ionic liquid dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Talanta 119:291–298. https://doi.org/10.1016/j.talanta.2013.11.023
Russo MV, Notardonato I, Cinelli G, Avino P (2012) Evaluation of an analytical method for determining phthalate esters in wine samples by solid-phase extraction and gas chromatography coupled with ion-trap mass spectrometer detector. Anal Bioanal Chem 402:1373–1381. https://doi.org/10.1007/s00216-011-5551-9
Cai YQ, Bin JG, Liu JF, Zhou QX (2003) Multi-walled carbon nanotubes packed cartridge for the solid-phase extraction of several phthalate esters from water samples and their determination by high performance liquid chromatography. Anal Chim Acta 494:149–156. https://doi.org/10.1016/j.aca.2003.08.006
Psillakis E, Kalogerakis N (2003) Hollow-fibre liquid-phase microextraction of phthalate esters from water. J Chromatogr A 999:145. https://doi.org/10.1016/S0021-9673(03)00390-X
Pang YH, Yue Q, Huang Y, Yang C, Shen XF (2020) Facile magnetization of covalent organic framework for solid-phase extraction of 15 phthalate esters in beverage samples. Talanta 206:120194. https://doi.org/10.1016/j.talanta.2019.120194
Wang X, Feng J, Tian Y, Li C, Ji X, Luo C, Sun M (2019) Melamine-formaldehyde aerogel functionalized with polydopamine as intube solid-phase microextraction coating for the determination of phthalate esters. Talanta 199:317–323. https://doi.org/10.1016/j.talanta.2019.02.081
Wu Y, Zhou Q, Yuan Y, Wang H, Tong Y, Zhan Y, Sheng X, Sun Y, Zhou X (2020) Enrichment and sensitive determination of phthalate esters in environmental water samples: a novel approach of MSPE-HPLC based on PAMAM dendrimers-functionalized magnetic-nanoparticles. Talanta 206:120213. https://doi.org/10.1016/j.talanta.2019.120213
Guo G, Chen G, Ma J, Jia Q (2019) A triazine based organic framework with micropores and mesopores for use in headspace solid phase microextraction of phthalate esters. Microchim Acta 186:4. https://doi.org/10.1007/s00604-018-3060-7
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2143 kb)
Rights and permissions
About this article
Cite this article
Aghaziarati, M., Yamini, Y. & Shamsayei, M. An electrodeposited terephthalic acid-layered double hydroxide (Cu-Cr) nanosheet coating for in-tube solid-phase microextraction of phthalate esters. Microchim Acta 187, 118 (2020). https://doi.org/10.1007/s00604-019-4102-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-4102-5