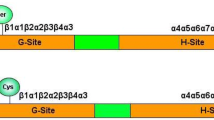
Abstract
High-molecular-weight glutenin subunits (HMW-GS) play an important role for the baking quality of wheat. The ancient wheats emmer and spelt differ in their HMW-GS pattern compared to modern common wheat and this might be one reason for their comparatively poor baking quality. The aim of this study was to elucidate similarities and differences in the amino acid sequences of two 1Bx HMW-GS of common wheat, spelt and emmer. First, the sodium dodecyl polyacrylamide gel electrophoresis (SDS-PAGE) system was optimized to separate common wheat, spelt and emmer Bx6 and Bx7 from other HMW-GS (e.g., 1Ax and 1By) in high concentrations. The in-gel digests of the Bx6 and Bx7 bands were analyzed by untargeted LC-MS/MS experiments revealing different UniProtKB accessions in spelt and emmer compared to common wheat. The HMW-GS Bx6 and Bx7, respectively, of emmer and spelt showed differences in the amino acid sequences compared to those of common wheat. The identities of the peptide variations were confirmed by targeted LC-MS/MS. These peptides can be used to differentiate between Bx6 and Bx7 of spelt and emmer and Bx6 and Bx7 of common wheat. The findings should help to increase the reliability and curation status of wheat protein databases and to understand the effects of protein structure on the functional properties.

Graphical abstract







Similar content being viewed by others
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) with the dataset identifier PXD016342 and are publicly available on Panorama Public (https://panoramaweb.org/a2lkrI.url).
References
Goesaert H, Brijs K, Veraverbeke WS, Courtin CM, Gebruers K, Delcour J. A. Wheat flour constituents: how they impact bread quality, and how to impact their functionality. Trends Food Sci Technol. 2005;16:12–30.
Osborne TB. The proteins of the wheat kernel. Washington: Carnegie Institution; 1907.
Wieser H. Chemistry of gluten proteins. Food Microbiol. 2007;24(2):115–9.
Payne PI. Genetics of wheat storage proteins and the effect of allelic variation on breadmaking quality. Annu Rev Plant Physiol. 1987;38:141–53.
Payne PI, Corfield G, Holt LM, Blackman JA. Correlation between the inheritance of certain high molecular weight subunits of glutenin and bread making quality in progenies of six crosses of bread wheat. J Sci Food Agric. 1981;32:51–60.
Payne PI, Law CN, Mudd EE. Control by homologous group 1 chromosomes of the high-molecular-weight subunits of glutenin, a major protein of wheat endosperm. Theor Appl Genet. 1980;58:113–20.
Payne PI, Lawrence GJ. Catalogue of alleles for the complex gene loci Glu-A1, Glu-B1 and Glu-D1 which code for high-molecular weight subunits of glutenin in hexaploid wheat. Cereal Res Commun. 1983;11:29–35.
Payne PI, Holt LM, Law CN. Structural and genetical studies on the high-molecular-weight subunits of wheat glutenin. Theor Appl Genet. 1981;60:229–36.
Jiang P, Xue J, Duan L, Gu Y, Mu J, Han S, et al. Effects of high-molecular-weight glutenin subunit combination in common wheat on the quality of crumb structure. J Sci Food Agric. 2019;99(4):1501–8.
Shewry PR, Halford NG, Tatham AS. High molecular weight subunits of wheat glutenin. J Cereal Sci. 1992;15(2):105–20.
Geisslitz S, Wieser H, Scherf KA, Koehler P. Gluten protein composition and aggregation properties as predictors for bread volume of common wheat, spelt, durum wheat, emmer and einkorn. J Cereal Sci. 2018;83:204–12.
Schalk K, Lexhaller B, Koehler P, Scherf KA. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS One. 2017;12(2):e0172819 1981;32:359–71.
Wieser H, Antes S, Seilmeier W. Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem. 1998;75(5):644–50.
Dong K, Hao C, Wang A, Cai M, Yan Y. Characterization of HMW glutenin subunits in bread and tetraploid wheats by reversed-phase high-performance liquid chromatography. Cereal Res Commun. 2009;37(1):65–73.
Qian Y, Preston K, Krokhin O, Mellish J, Ens W. Characterization of wheat gluten proteins by HPLC and MALDI TOF mass spectrometry. J Am Soc Mass Spectrom. 2008;19(10):1542–50.
Marchylo BA, Kruger JE, Hatcher DW. Quantitative reversed-phase high-performance liquid chromatographic analysis of wheat storage proteins as a potential quality prediction tool. J Cereal Sci. 1989;9(2):113–30.
Lagrain B, Brunnbauer M, Rombouts I, Koehler P. Identification of intact high molecular weight glutenin subunits from the wheat proteome using combined liquid chromatography-electrospray ionization mass spectrometry. PLoS One. 2013;8(3):e58682.
Gao L, Ma W, Chen J, Wang KE, Li J, Wang S, et al. Characterization and comparative analysis of wheat high molecular weight glutenin subunits by SDS-PAGE, RP-HPLC, HPCE, and MALDI-TOF-MS. J Agric Food Chem. 2010;58(5):2777–86.
Zhang Q, Dong Y, An X, Wang A, Zhang Y, Li X, et al. Characterization of HMW glutenin subunits in common wheat and related species by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). J Cereal Sci. 2008;47(2):252–61.
Liu L, Wang A, Appels R, Ma J, Xia X, Lan P, et al. A MALDI-TOF based analysis of high molecular weight glutenin subunits for wheat breeding. J Cereal Sci. 2009;50(2):295–301.
Jin M, Xie ZZ, Ge P, Li J, Jiang SS, Subburaj S, et al. Identification and molecular characterisation of HMW glutenin subunit 1By16* in wild emmer. J Appl Genet. 2012;53(3):249–58.
Barro F, Rooke L, Békés F, Gras P, Tatham AS, Fido R, et al. Transformation of wheat with high molecular weight subunit genes results in improved functional properties. Nat Biotechnol. 1997;15(12):1295–9.
Blatter RHE, Jacomet S, Schlumbaum A. About the origin of European spelt (Triticum spelta L.): allelic differentiation of the HMW Glutenin B1-1 and A1-2 subunit genes. Theor Appl Genet. 2004;108:360.
Dupont FM, Vensel WH, Tanaka CK, Hurkman WJ, Altenbach SB. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci. 2011;9:10.
Colgrave ML, Byrne K, Howitt CA. Food for thought: selecting the right enzyme for the digestion of gluten. Food Chem. 2017;234:389–97.
Oezbek O, Goeçmen Taşkin B, Keskin Şan S, Eser V, Arslan O. High-molecular-weight glutenin subunit variation in Turkish emmer wheat [Triticum turgidum L. ssp. dicoccon (Schrank) Thell.] landraces. Plant Syst Evol. 2012;298:1795–804.
Pflueger LA, Martín LM, Alvarez JB. Variation in the HMW and LMW glutenin subunits from Spanish accessions of emmer wheat (Triticum turgidum ssp. Dicoccum Schrank). Theor Appl Genet. 2001;102:767–72.
Kasarda DD, Woodard KM, Adalsteins AE. Resolution of high molecular weight glutenin subunits by a new SDS-PAGE system incorporating a neutral pH buffer. Cereal Chem. 1998;75:70e71.
Lagrain B, Rombouts I, Wieser H, Delcour JA, Koehler P. A reassessment of the electrophoretic mobility of high molecular weight glutenin subunits of wheat. J Cereal Sci. 2012;56:726–32.
Shevchenko A, Tomas H, Havlis J, Olsen JV, Mann M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc. 2006;1(6):2856–60.
Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–805.
Ma B, Zhang K, Hendrie C, Liang C, Li M, Doherty-Kirby A, et al. PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17(20):2337–42.
MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–8.
Gessendorfer B, Koehler P, Wieser H. Preparation and characterization of enzymatically hydrolyzed prolamins from wheat, rye, and barley as references for the immunochemical quantitation of partially hydrolyzed gluten. Anal Bioanal Chem. 2009;395(6):1721–8.
Scarnato L, Gadermaier G, Volta U, De Giorgio R, Caio G, Lanciotti R, et al. Immunoreactivity of gluten-sensitized sera toward wheat, rice, corn, and amaranth flour proteins treated with microbial transglutaminase. Front Microbiol. 2019;10:470.
Wieser H, Scherf KA. Preparation of a defined gluten hydrolysate for diagnosis and clinical investigations of wheat hypersensitivities. Nutrients. 2018;10:1411.
Wan Y, Gritsch CS, Hawkesford MJ, Shewry PR. Effects of nitrogen nutrition on the synthesis and deposition of the omega-gliadins of wheat. Ann Bot. 2014;113(4):607–15.
Pastorello EA, Farioli L, Conti A, Pravettoni V, Bonomi S, Iametti S, et al. Wheat IgE-mediated food allergy in European patients: α-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins. Int Arch Allergy Immunol. 2007;144(1):10–22.
Marchetti-Deschmann M, Lehner A, Peterseil V, Soevegjarto F, Hochegger R, Allmaier G. Fast wheat variety classification by capillary gel electrophoresis-on-a-chip after single-step one-grain high molecular weight glutenin extraction. Anal Bioanal Chem. 2011;400:2403–14.
Ludwig C, Aebersold R. Getting absolute: determining absolute protein quantities via selected reaction monitoring mass spectrometry. In: Eyers CE, Gaskell S, editors. Quantitative Proteomics. London: The Royal Society of Chemistry; 2014. pp. 80–109.
Bogdanow B, Zauber H, Selbach M. Systematic errors in peptide and protein identification and quantification by modified peptides. Mol Cell Proteomics. 2016;15:2791–801.
Rechenberger J, Samaras P, Jarzab A, Behr J, Frejno M, Djukovic A, et al. Challenges in clinical metaproteomics highlighted by the analysis of acute leukemia patients with gut colonization by multidrug-resistant Enterobacteriaceae. Proteomes. 2019;7:2.
The International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:eaar7191.
Payne PI, Nightingale MA, Krattiger AF, Holt LM. The relationship between HMW glutenin subunit composition and the bread-making quality of British-grown wheat varieties. J Sci Food Agric. 1987;40:51–65.
Acknowledgments
The authors thank Sami Kaviani-Nejad and Katharina Booz (Leibniz-LSB@TUM) and Bert Schipper (Wageningen UR) for excellent technical assistance.
Funding
This IGF Project of the FEI was supported via AiF within the programme for promoting the Industrial Collective Research (IGF) of the German Ministry of Economic Affairs and Energy (BMWi), based on a resolution of the German Parliament. Project AiF 18355 N. This submission was supported by the Fachgruppe Analytische Chemie of the Gesellschaft Deutscher Chemiker via a scholarship for finalizing a publication in a cooperation project. Additional funding came from the Technical University of Munich (TUM) through the TUM Graduate School (GS) Internationalization Grant.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimental work, data analysis, and preparation of tables and figures were performed by Sabrina Geisslitz. Data analysis was additionally done by Antoine H. P. America. The first draft of the manuscript was written by Sabrina Geisslitz and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
No human or animal subjects were used in the study. No informed consent was required for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1129 kb)
Rights and permissions
About this article
Cite this article
Geisslitz, S., America, A.H.P. & Scherf, K.A. Mass spectrometry of in-gel digests reveals differences in amino acid sequences of high-molecular-weight glutenin subunits in spelt and emmer compared to common wheat. Anal Bioanal Chem 412, 1277–1289 (2020). https://doi.org/10.1007/s00216-019-02341-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02341-9