Abstract
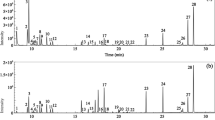
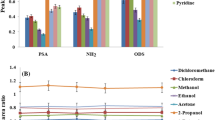
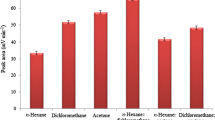
Deep eutectic solvents (DESs) were investigated as extracting solvent for headspace single-drop microextraction (HS-SDME). The extraction efficiency of 10 DESs mainly composed of tetrabutylammonium bromide (N4444Br) and long-chain alcohols was evaluated for the extraction of terpenes from six spices (cinnamon, cumin, fennel, clove, thyme, and nutmeg). The DES composed of N4444Br and dodecanol at a molar ratio of 1:2 showed the highest extraction efficiency and was selected to conduct the extractions of terpenes in the rest of the study. HS-SDME was optimized by design of experiments. Only two parameters from the four studied showed a significant influence on the efficiency of the method: the extraction time and the extraction temperature. The optimal extraction conditions were determined by response surface methodology. All extracts were analyzed by gas chromatography coupled to mass spectrometry (GC-MS). More than 40 terpenes were extracted and identified in nutmeg, the richest extract in terpenes in this study. Quantitative analysis based on 29 standards was conducted for each extract. Good linearity was obtained for all standards (R2 > 0.99) in the interval of 1 to 500 μg/g. Limits of quantification ranged from 0.47 μg/g (borneol) to 86.40 μg/g (α-farnesene) with more than half of the values under 2 μg/g. HS-SDME is simple, rapid, and cheap compared with conventional extraction methods. The use of DESs makes this extraction method “greener” and it was shown that DESs can be suitable solvents for the extraction of bioactive compounds from plants.





Similar content being viewed by others
References
Newman DJ, Cragg GM, Kingston DGI. Natural products as pharmaceuticals and sources for lead structures. In: The practice of medicinal chemistry, Second Edi. Elsevier Inc., 2003; pp 91–109.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77.
Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006;9:1–8.
Croteau R, Kutchan TM, Lewis NL. Natural products (secondary metabolites). In: Biochemistry and molecular biology of plants. 2000;, pp 1250–1318.
Vavitsas K, Fabris M, Vickers CE. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes (Basel). 2018;9:1–19.
De Almeida RN, De Fátima AM, Maior FNS, De Sousa DP. Essential oils and their constituents: anticonvulsant activity. Molecules. 2011;16:2726–42.
Bart H-J. Extraction of natural products from plants – an introduction. In: Industrial scale natural products extraction. 2011;, pp 1–25.
Kozioł A, Stryjewska A, Librowski T, Sałat K, Gaweł M, Moniczewski A, et al. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini-Reviews Med Chem. 2014;14:1156–68.
Fonsêca DV, Salgado PRR, de Aragão Neto HC, Golzio AMFO, Caldas Filho MRD, Melo CGF, et al. Ortho-eugenol exhibits anti-nociceptive and anti-inflammatory activities. Int Immunopharmacol. 2016;38:402–8.
Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005;49:2474–8.
Salakhutdinov NF, Volcho KP, Yarovaya OI. Monoterpenes as a renewable source of biologically active compounds. Pure Appl Chem. 2017;89:1105–17.
Yano S, Suzuki Y, Yuzurihara M, Kase Y, Takeda S, Watanabe S, et al. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur J Pharmacol. 2006;553:99–103.
Yang C, Wang J, Li D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: a review. Anal Chim Acta. 2013;799:8–22.
Theis AL, Waldack AJ, Hansen SM, Jeannot MA. Headspace solvent microextraction. Anal Chem. 2001;73:5651–4.
Wardencki W, Curyło J, Namieśnik J. Trends in solventless sample preparation techniques for environmental analysis. J Biochem Biophys Methods. 2007;70:275–88.
Clark KD, Emaus MN, Varona M, Bowers AN, Anderson JL. Ionic liquids: solvents and sorbents in sample preparation. J Sep Sci. 2018;41:1–416.
Zainal-Abidin MH, Hayyan M, Hayyan A, Jayakumar NS. New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Anal Chim Acta. 2017;979:1–23.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride urea mixtures. Chem Commun. 2003:70–1.
Zhang Q, De Oliveira VK, Royer S, François J. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41:7108–46.
Yousefi SM, Shemirani F, Ghorbanian SA. Enhanced headspace single drop microextraction method using deep eutectic solvent based magnetic bucky gels: application to the determination of volatile aromatic hydrocarbons in water and urine samples. J Sep Sci. 2018;41:966–74.
Jeong KM, Jin Y, Yoo DE, Han SY, Kim EM, Lee J. One-step sample preparation for convenient examination of volatile monoterpenes and phenolic compounds in peppermint leaves using deep eutectic solvents. Food Chem. 2018;251:69–76.
Su E, Yang M, Cao J, Lu C, Wang J, Cao F. Deep eutectic solvents as green media for efficient extraction of terpene trilactones from Ginkgo biloba leaves. J Liq Chromatogr Relat Technol. 2017;40:1–7.
Cao J, Chen L, Li M, Cao F, Zhao L, Su E. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018;20:1879–86.
Tang B, Bi W, Zhang H, Ho RK. Deep eutectic solvent-based HS-SME coupled with GC for the analysis of bioactive terpenoids in Chamaecyparis obtusa leaves. Chromatographia. 2014;77:373–7.
Tang W, Dai Y, Row KH. Evaluation of fatty acid/alcohol-based hydrophobic deep eutectic solvents as media for extracting antibiotics from environmental water. Anal Bioanal Chem. 2018;410:7325–36.
Candioti LV, De Zan MM, Cámara MS, Goicoechea HC. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta. 2014;124:123–38.
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–77.
Lundstedt T, Seifert E, Abramo L, Thelin B, Åsa N, Pettersen J, et al. Experimental design and optimization. Chemom Intell Lab Syst. 1998;42:3–40.
Espino M, de los Ángeles Fernández M, Gomez FJV, Silva MF. Natural designer solvents for greening analytical chemistry. TrAC - Trends Anal Chem. 2016;76:126–36.
Simpson GIC, Jackson YA. Comparison of the chemical composition of East Indian, Jamaican and other West Indian essential oils of Myristical fragrans Houtt. J Essent Oil Res. 2002;14:6–9.
Tang B, Row KH. Recent developments in deep eutectic solvents in chemical sciences. Monatsh Chem. 2013;144:1427–54.
Tang B, Tian M, Row KH. Determination of terpenoids in Chamaecyparis obtusa leaves by headspace single-drop microextraction with gas chromatography detection. Anal Lett. 2014;47:48–57.
Sawaddipanich V, Chanthai S. Headspace-single drop microextraction followed by gas chromatographic determination of key aroma compounds in tomato fruits and their sample products. Orient J Chem. 2016;32:1271–82.
Moradi M, Kaykhaii M, Ghiasvand AR, Shadabi S, Salehinia A. Comparison of headspace solid-phase microextraction, headspace single-drop microextraction and hydrodistillation for chemical screening of volatiles in Myrtus communis L. Phytochem Anal. 2011;23:379–86.
Jiang C, Wei S, Li X, Zhao Y, Shao M, Zhang H, et al. Ultrasonic nebulization headspace ionic liquid-based single drop microextraction of flavour compounds in fruit juices. Talanta. 2013;106:237–42.
Jalali Heravi M, Sereshti H. Determination of essential oil components of Artemisia haussknechtii Boiss. Using simultaneous hydrodistillation-static headspace liquid phase microextraction-gas chromatography mass spectrometry. J Chromatogr A. 2007;1160:81–9.
Sadeghian F, Ebrahimi P, Shakeri A, Jamali MR. Extraction of Citrus paradisi volatile components by headspace single-drop microextraction and statistical modeling. J Chromatogr Sci. 2016;54:1263–9.
Marongiu B, Piras A, Porcedda S, Tuveri E, Sanjust E, Meli M, et al. Supercritical CO2 extract of Cinnamomum zeylanicum: chemical characterization and antityrosinase activity. J Agric Food Chem. 2007;55:10022–7.
Yang J, Wei H, Yu C, Shi Y, Zhang H. Extraction of the volatile and semivolatile compounds in seeds of Cuminum cyminum L. using hydrodistillation followed by headspace-ionic liquid-based single-drop microextraction. Chromatographia. 2012;75:1435–43.
Santana De Oliveira M, Almeida Da Costa W, Santiago Pereira D, Santos Botelho R, Oliveira De Alencar Menezes T, Helena De Aguiar Andrade E, et al. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J Supercrit Fluids. 2016;118:185–93.
Dawidowicz AL, Rado E, Wianowska D, Mardarowicz M, Gawdzik J. Application of PLE for the determination of essential oil components from Thymus vulgaris L. Talanta. 2008;76:878–84.
Piras A, Rosa A, Marongiu B, Atzeri A, Dessì MA, Falconieri D, et al. Extraction and separation of volatile and fixed oils from seeds of myristica fragrans by supercritical CO2: chemical composition and cytotoxic activity on caco-2 cancer cells. J Food Sci. 2012;77:1–6.
Diao W-R, Hu Q-P, Zhang H, Xu J-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014;35:109–16.
Funding
This work has been financially supported by “Association Nationale Recherche Technologie” with the CIFRE Contract No. 2016/0447.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 222 kb)
Rights and permissions
About this article
Cite this article
Triaux, Z., Petitjean, H., Marchioni, E. et al. Deep eutectic solvent–based headspace single-drop microextraction for the quantification of terpenes in spices. Anal Bioanal Chem 412, 933–948 (2020). https://doi.org/10.1007/s00216-019-02317-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02317-9