Abstract
Background
The transmembrane 6 superfamily member 2 (TM6SF2) E167K polymorphism influences estimated glomerular filtration rate (eGFR) in adults without diabetes and without obesity. We aimed exploring the impact of this polymorphism on eGFR in children with obesity with and without non-alcoholic fatty liver disease (NAFLD).
Methods
We genotyped 531 children with obesity for TM6SF2 E167K polymorphism. NAFLD was defined by ultrasound detected liver steatosis and/or ALT > 40 IU/L.
Results
Patients carrying the TM6SF2 167K allele showed higher eGFR levels compared with E167 homozygous patients both among subjects with and without NAFLD. A general linear model confirmed a direct and significant association of eGFR values with TM6SF2 genotype both in patients with and without NAFLD. This association, however, was stronger in patients with NAFLD.
Conclusions
Children with obesity carrying the TM6SF2 167K allele show higher eGFR levels compared with E167 allele homozygous subjects, independently of NAFLD. A major effect of this polymorphism in the presence of NAFLD was captured.
Similar content being viewed by others
Introduction
Obesity can affect renal function already in childhood and, more in detail, a positive correlation of estimated glomerular filtration rate (eGFR) with body mass index (BMI) and a negative correlation of eGFR with duration of obesity exist.1 Similarly, non-alcoholic fatty liver disease (NAFLD) has been associated with increased prevalence of chronic kidney disease.2 Recently, a possible genetic link between NAFLD and renal function in children has been demonstrated. Targher et al. showed that children with NAFLD and homozygous for the rare allele of the rs738409 patatin like phospholipase containing domain 3 (PNPLA3) polymorphism, which predisposes to NAFLD, had lower eGFR and higher 24-h proteinuria compared to other genotypes.3 Similarly, our group showed that children with obesity and homozygous for PNPLA3 rare allele had lower eGFR levels compared to other genotypes both among patients with and without NAFLD, with a major effect of this polymorphism in the presence of NAFLD.4
As rs738409 PNPLA3 polymorphism, another important determinant of NAFLD in children is the transmembrane 6 superfamily member 2 (TM6SF2) E167K polymorphism, with TM6SF2 167K allele predisposing to NAFLD.5 This allele has both a deleterious effect on the liver determining reduced very low-density lipoprotein secretion and subsequently fatty liver and a positive action on cardiovascular risk reducing serum low-density lipoprotein and triglyceride levels.5 Therefore, the 167K allele in the TM6SF2 gene has been suggested to protect against cardiovascular disease at the cost of developing NAFLD in adults.6,7,8 TM6SF2 E167K polymorphism modulates eGFR in adults without obesity and diabetes with the 167K allele determining higher eGFR levels.9 Therefore, we hypothesized that children with obesity carrying the TM6SF2 167K allele—despite being at higher risk of NAFLD—could have higher eGFR levels compared with E167 homozygous patients. We aimed exploring the impact of TM6SF2 E167K polymorphisms on eGFR levels in children with obesity with and without NAFLD and without evidence of primary kidney disease.
Materials and methods
Cohort description and clinical evaluation
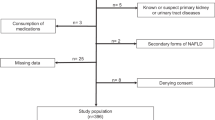
All the children and adolescents consecutively attending our Department between January 2016 and January 2019 were enrolled. Inclusion criteria were BMI >95th percentile according to reference values10 and eGFR > 90 mL/min/1.73 m2. Patients with secondary forms of NAFLD, secondary forms of obesity, consumption of any medication, eGFR < 90 mL/min/1.73 m2, presence of proteinuria or hematuria at urine dipstick on urine samples correctly collected,11 missing eGFR levels, or denying consent to undergo diagnostic procedures were excluded from the study (Fig. 1). We excluded patients with reduced eGFR and proteinuria/hematuria at urine dipstick because they could present a possible underlining primary kidney disease potentially affecting our analysis. The ethical committee of Università degli Studi della Campania “Luigi Vanvitelli” approved the study. A written informed consent was obtained before any procedure. Weight, waist circumference, height, waist-to-height ratio, BMI standard deviation score (BMI-SDS), and pubertal stage were obtained as previously described.1 Patients’ growth charts were evaluated to calculate duration of obesity.
Laboratory and ultrasound evaluation
Serum insulin, glucose, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, alanine transaminase (ALT), and aspartate transaminase (AST) were assayed and homeostasis model assessment insulin resistance (HOMA-IR) was calculated.12 Serum creatinine (mg/dL) was always measured with Jaffè method and the eGFR calculated by using the original Schwartz equation.13 We normalized the eGFR to the ideal body weight-derived body surface area.1
The presence of steatosis was detected using ultrasound imaging. Liver steatosis was assessed as present or absent.14 NAFLD was defined by the presence of ultrasound-detected liver steatosis and/or ALT levels > 40IU/L.
Genotyping
At diagnosis, informed consent was collected for DNA extraction from peripheral whole blood. Genomic DNA was extracted from peripheral whole blood with a DNA extraction kit (Promega, Madison, WI, USA). Patients were genotyped for the TM6SF2 E167K (rs58542926) polymorphism by Taqman allelic discrimination assay on ABI 7900HT Real Time PCR system. Particularly, pre-designed assay primers and probes were purchased from Applied Biosystems (Foster City, CA, USA) to analyze the c.499A > G polymorphism underlying the p.Glu167Lys substitution of the TM6SF2 gene.
Statistical analysis
A Chi-Square test was used to verify whether the genotypes were in Hardy–Weinberg equilibrium and to compare categorical variables. We classified the population according to the presence/absence of NAFLD and we compared eGFR levels in these two groups (with and without NAFLD) on the basis of TM6SF2 E167K polymorphism.
Differences for continuous variables were analyzed using analysis of variance (ANOVA) if normally distributed or using Kruskal–Wallis test if non-normally distributed. The non-normally distributed variables were ALT, AST, total cholesterol, LDL-C, HDL-C, triglycerides, glycemia, and HOMA-IR, but raw means are shown.
Only one patient was homozygous for the rare allele, therefore the genotype was coded with an additive model of inheritance. The genotype was coded 0 or 1 corresponding to the subjects homozygous for the wild-type allele and carrying the rare allele, respectively.
A general linear model (GLM) for eGFR variance including gender, duration of obesity, TM6SF2 genotype, BMI-SDS, HOMA, LDL, and triglycerides was made both in patients with and without NAFLD. The non-normally distributed variables were log transformed for this analysis.
A comparison of regression lines by ANOVA was performed to examine the influence of TM6SF2 genotype on the relationship between eGFR and age both among patients with and without NAFLD.
The IBM SPSS Statistics software, Version 24 (IBM, Armonk, NY) was used for all statistical analyses. Data were expressed as means ± SD. p Values <0.05 were considered statistically significant.
Results
We enrolled 531 patients with obesity. The mean age was 10.86 ± 2.96 years, 52.5% of the patients were of male gender, and the mean BMI-SDS was 2.97 ± 0.79.
The frequency of the TM6SF2 E167K polymorphism distribution was in Hardy–Weinberg equilibrium (p > 0.05).
Patients carrying the TM6SF2 167K allele showed higher eGFR levels compared with TM6SF2 E167 homozygous (wild type) patients both among subjects with and without NAFLD (Table 1).
A GLM for eGFR variance including gender, duration of obesity, TM6SF2 genotype, BMI-SDS, HOMA, LDL, and triglycerides confirmed a direct and significant association of eGFR values with TM6SF2 genotype both in patients with and without NAFLD (Table 1).
A comparison of regression line slopes was performed to examine the influence of TM6SF2 genotype on the relationship between eGFR and age both among patients with and without NAFLD. TM6SF2 E167 homozygous patients with NAFLD showed a significantly higher decline of eGFR with the increase of age compared with the patients with NAFLD carrying the TM6SF2 167K allele (p value for intercepts = 0.8; p value for slopes = 0.04; Fig. 2a). We observed a similar trend among patients without NAFLD (p value for intercepts = 0.53; p value for slopes = 0.09; Fig. 2b).
Regression analysis describing the relationship between eGFR and age according to TM6SF2 genotype in patients with (a) and without (b) NAFLD. a The regression is described by the equation y = 234.274 − 5.32123 × x − 43.3706 × (tm6sf2 = 1) + 3.96634 × x × (tm6sf2 = 1). p Value for intercepts is 0.80 while for slopes it is 0.04. b The regression is described by the equation y = 259.285 − 8.10944 × x − 50.2069 × (tm6sf2 = 1) + 3.99784 × x × (tm6sf2 = 1). p Value for intercepts is 0.53 while for slopes it is 0.09.
Discussion
In this paper, we captured an effect of the TM6SF2 E167K polymorphism on renal function in a population of children with obesity without evidence of primary kidney disease. In children with obesity, the patients carrying the TM6SF2 167K allele presented higher eGFR levels compared with the homozygous subjects for the TM6SF2 E167 allele. We confirmed these findings both in patients with and without NAFLD. Therefore, we can hypothesize that the effect of this polymorphism on renal function is independent of NAFLD, although in NAFLD patients we observed a stronger association between TM6SF2 E167K polymorphism and eGFR (Table 1).
Previously, this association had been investigated only in a letter by Musso et al. that demonstrated both in adults with and without NAFLD that TM6SF2 167K allele was associated with higher eGFR levels and with lower prevalence of albuminuria and chronic kidney disease.9 In other words, these findings further supported that TM6SF2 might act as a switch gene able to disconnect the risk of NAFLD from cardiovascular risk.
Our study added to the evidence by Musso et al.9 that the TM6SF2 167K allele might protect against decline of renal function increasing with age. In fact, children with NAFLD and homozygous for the TM6SF2 E167 allele showed a significantly higher decline of eGFR increasing with age compared with children with NAFLD carrying the TM6SF2 167K allele (Fig. 2a). We found a similar trend among the children without NAFLD (Fig. 2b). This is in line with the lower prevalence of chronic kidney disease among the carriers of the TM6SF2 167K allele in adults.9
The TM6SF2 gene is expressed in the small intestine, liver, and kidney and encodes for a protein of 351 amino acids with 7–10 predicted transmembrane domains. The TM6SF2 167K allele has been associated with higher hepatic triglyceride content (HTGC), with higher serum levels of ALT and lower plasma levels of liver-derived triglyceride-rich lipoproteins. The hypothesized TM6SF2 protein function appears to be the promotion of very low-density lipoprotein (VLDL) secretion and probably the increased HTGC result from a reduction in TM6SF2 protein function. Therefore, the role of the TM6SF2 167K allele in the NAFLD pathophysiology is represented by a reduced VLDL secretion that could explain the higher HTGC, in turn resulting in higher ALT levels and in lower serum LDL-C and triglycerides levels. In addition, evidence suggests that TM6SF2 167K allele could reduce post-prandial intestinal absorption of lipids in the small intestine.15 Because there is evidence of a renal uptake of lipids that can play a role in renal damage throughout lipotoxicity,16,17,18 we can speculate that, similarly to small intestine, the TM6SF2 167K allele could reduce uptake of lipids in the glomerular and tubular cells protecting kidneys against damage. Therefore, the pathophysiological mechanism by which the TM6SF2 E167K polymorphism could improve renal function might be principally mediated by decrease of circulating levels of atherogenic lipoproteins with subsequent protection against cardiovascular disease and by reduced lipotoxicity.5,16,17,18
Moreover, we hypothesize that the effect of the TM6SF2 167K allele on renal function is amplified in the presence of NAFLD because NAFLD is associated with increased cardiovascular risk,19 permitting, therefore, a magnification of the “renal protective effect” of the TM6SF2 167K allele.
Limitations of our study are represented by the lack of a more accurate method to measure GFR (i.e., by cystatin C). The original Schwartz equation is clinically easy to use because the only measures required are height and serum creatinine.13 However, the use of this formula is not suggested when creatinine levels are dosed with isotope dilution mass spectrometry traceable method.20 In this case, in fact, the use of original Schwartz equation could overestimate GFR by 20–40%21 and another equation has been proposed.22 Because our laboratories use the Jaffè method to dose creatinine levels, we used the original Schwartz equation to calculate eGFR.13 Nevertheless, in children with mildly decreased or normal kidney function and in older adolescents with normal and a8bnormal kidney function, these equations need further validation to inform clinical use.20 Another limitation is the diagnosis of NAFLD by ultrasound and ALT levels and not by liver biopsy or magnetic resonance.23 Moreover, this is a pathophysiological study evaluating only children with normal renal function.
In conclusion, our observation adds to the current knowledge showing that, in addition to the rs738409 PNPLA3 polymorphism, also the TM6SF2 E167K polymorphism could have an effect on renal function strengthening the evidence of the relationship between NAFLD and kidney function already in childhood.
References
Marzuillo, P. et al. Anthropometric and biochemical determinants of estimated glomerular filtration rate in a large cohort of obese children. J. Ren. Nutr. 28, 359–362 (2018).
Targher, G. & Byrne, C. D. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 13, 297–310 (2017).
Targher, G. et al. Relationship between PNPLA 3 rs738409 polymorphism and decreased kidney function in children with NAFLD. Hepatology 70, 142–153 (2019).
Marzuillo, P. et al. Nonalcoholic fatty liver disease and eGFR levels could be linked by the PNPLA3 I148M polymorphism in children with obesity. Pediatr. Obes. 14, e12539 (2019).
Marzuillo, P. et al. Understanding the pathophysiological mechanisms in the pediatric non-alcoholic fatty liver disease: the role of genetics. World J. Hepatol. 7, 1439 (2015).
Kozlitina, J. et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 46, 352–356 (2014).
Dongiovanni, P. et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology 61, 506–514 (2015).
Holmen, O. L. et al. Systematic evaluation of coding variation identifies a candidate causal variant in TM6SF2 influencing total cholesterol and myocardial infarction risk. Nat. Genet. 46, 345–351 (2014).
Musso, G., Cassader, M. & Gambino, R. PNPLA3 rs738409 and TM6SF2 rs58542926 gene variants affect renal disease and function in nonalcoholic fatty liver disease. Hepatology 62, 658–659 (2015).
Cacciari, E. et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Invest. 29, 581–593 (2006).
Marzuillo, P. et al. Cleaning the genitalia with plain water improves accuracy of urine dipstick in childhood. Eur. J. Pediatr. 177, 1573–1579 (2018).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and ß-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Schwartz, G. J., Brion, L. P. & Spitzer, A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr. Clin. North Am. 34, 571–590 (1987).
Marzuillo, P. et al. Novel association between a nonsynonymous variant (R270H) of the G-protein-coupled receptor 120 and liver injury in children and adolescents with obesity. J. Pediatr. Gastroenterol. Nutr. 59, 472–475 (2014).
Li, T.-T. et al. TM6SF2: a novel target for plasma lipid regulation. Atherosclerosis 268, 170–176 (2018).
Moestrup, S. K. & Nielsen, L. B. The role of the kidney in lipid metabolism. Curr. Opin. Lipidol. 16, 301–306 (2005).
Abrass, C. K. Lipid metabolism and renal disease. Contrib. Nephrol. 151, 106–121 (2006).
Bobulescu, I. A. Renal lipid metabolism and lipotoxicity. Curr. Opin. Nephrol. Hypertens. 19, 393–402 (2010).
Patil, R. & Sood, G. K. Non-alcoholic fatty liver disease and cardiovascular risk. World J. Gastrointest. Pathophysiol. 8, 51–58 (2017).
Fadrowski, J. J. & Furth, S. L. GFR estimation in children: questions and answers (and questions). Clin. J. Am. Soc. Nephrol. 6, 1810–1812 (2011).
Srivastava, T., Alon, U. S., Althahabi, R. & Garg, U. Impact of standardization of creatinine methodology on the assessment of glomerular filtration rate in children. Pediatr. Res. 65, 113–116 (2009).
Schwartz, G. J. et al. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 20, 629–637 (2009).
Vajro, P. et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 54, 700–713 (2012).
Author information
Authors and Affiliations
Contributions
Research idea and study design: P.M., G.C., and E.M.d.G.; data acquisition: S.G., G.R.U. and A.D.S.; molecular analysis: G.C., G.R.U.; data analysis/interpretation: P.M., E.M.d.G., A.L.M.; statistical analysis: A.D.S., G.C. and M.P.; supervision or mentorship: E.M.d.G., P.M. and S.G. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.5 Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marzuillo, P., Di Sessa, A., Cirillo, G. et al. Transmembrane 6 superfamily member 2 167K allele improves renal function in children with obesity. Pediatr Res 88, 300–304 (2020). https://doi.org/10.1038/s41390-020-0753-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0753-5
This article is cited by
-
Kidney damage predictors in children with metabolically healthy and metabolically unhealthy obesity phenotype
International Journal of Obesity (2023)
-
Association between non-alcoholic fatty liver disease and subclinical hypothyroidism in children with obesity
Journal of Endocrinological Investigation (2023)
-
Association of metabolic dysfunction-associated fatty liver disease with kidney disease
Nature Reviews Nephrology (2022)