Abstract
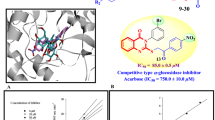
A series of new pyrrolidine-appended phenoxy-substituted quinoline derivatives were synthesized using 2-chloro-3-formyl quinoline. Initially, the second position of 2-chloro-3-formyl quinoline was successfully converted into various substituted phenoxy-substituted quinolines using various substituted phenols; then, its aldehyde function was reduced to its corresponding alcohols which is in turn converted into its corresponding pyrrolidine-appended phenoxy-substituted quinolines by treating it with 1-(2-chloroacetyl)pyrrolidine-2-carbonitrile. All these newly synthesized compounds were subjected to the in silico studies with the α-amylase and α-glucosidase enzymes to predict the binding affinity.







Similar content being viewed by others
References
M.A. Kazaz, V. Desseaux, G. Marchis-Mouren, E. Prodanov, M. Santimone, Eur. J. Biochem. 252, 100 (1998)
A.J. Hirsh, S.Y.M. Yao, J.D. Young, C.I. Cheeseman, Gastroenterology 113, 205 (1997)
S. Murugavel, C.S.J.P. Stephen, R. Subashini, D. Ananthakrishnan, J. Photochem. Photobiol. B 173, 216 (2017)
S. Sarveswari, V. Vijayakumar, Arab. J. Chem. 9, S35 (2016)
G.L. Balaji, K. Rajesh, S.K. Ali, V. Vijayakumar, Res. Chem. Intermed. 39, 1807 (2013)
G.L. Balaji, K. Rajesh, R. Priya, P. Iniyavan, R. Siva, V. Vijayakumar, Med. Chem. Res. 22, 3185 (2013)
K. Rajesh, B.P. Reddy, S. Sarveswari, V. Vijayakumar, Res. Chem. Intermed. 39, 4259 (2013)
K. Balamurugan, V. Jeyachandran, S. Perumal, T.H. Manjashetty, P. Yogeeswari, D. Sriram, Eur. J. Med. Chem. 45, 682 (2010)
K.D. Thomas, A.V. Adhikari, I.H. Chowdhury, T. Sandeep, R. Mahmood, B. Bhattacharya, E. Sumesh, Eur. J. Med. Chem. 46, 4834 (2011)
V. Ramesh, B.A. Rao, P. Sharma, B. Swarna, D. Thummuri, K. Srinivas, V.G.M. Naidu, V.J. Rao, Eur. J. Med. Chem. 83, 569 (2014)
S. Vandekerckhove, M. D’hooghe, Bioorg. Med. Chem. 23, 5098 (2015)
Y.-Q. Hu, C. Gao, S. Zhang, L. Xu, Z. Xu, L.-S. Feng, X. Wu, F. Zhao, Eur. J. Med. Chem. 139, 22 (2017)
Y.-L. Fan, X.-W. Cheng, J.-B. Wu, M. Liu, F.-Z. Zhang, Z. Xu, L.-S. Feng, Eur. J. Med. Chem. 146, 1 (2018)
T. Mahalakshmi, P. Lavanya, K.M. Kumar, V. Vijayakumar, S. Sarveswari, A. Anand, R. Sudha, J. Biomol. Struct. Dyn. 33, 961 (2015)
S. Sarveswari, V. Vijayakumar, R. Siva, R. Priya, Appl. Biochem. Biotechnol. 175, 43 (2015)
L. Jyothish Kumar, V. Vijayakumar, Res. Chem. Intermed. 43, 5691 (2017)
H. Maezaki, Y. Banno, Y. Miyamoto, Y. Moritou, T. Asakawa, O. Kataoka, K. Takeuchi, N. Suzuki, K. Ikedo, T. Kosaka, M. Sasaki, S. Tsubotani, A. Tani, M. Funami, Y. Yamamoto, M. Tawada, K. Aertgeerts, J. Yano, S. Oi, Bioorg. Med. Chem. 19, 4482 (2011)
D. Edmont, R. Rocher, C. Plisson, J. Chenault, Bioorg. Med. Chem. Lett. 10, 1831 (2000)
L. Jyothish Kumar, Y. Suresh, R. Rajasekaran, S. Rajeswara Reddy, V. Vijayakumar, J. Iran Chem. Soc. 16, 1071 (2019)
H. Nikookar, M. Mohammadi-Khanaposhtani, S. Imanparast, M.A. Faramarzic, P.R. Ranjbar, M. Mahdavi, B. Larijani, Bioorg. Chem. 77, 280 (2018)
M. Taha, M.T. Javid, S. Imran, M. Selvaraj, S. Chigurupati, H. Ullah, F. Rahim, F. Khan, J.I. Mohammad, K.M. Khan, Bioorg. Chem. 74, 179 (2017)
H.-W. Lee, J.-Y. Yang, H.-S. Lee, J. Korean Soc. Appl. Biol. Chem. 57, 441 (2014)
H. Bischoff, Clin. Invest. Med. 18, 303 (1995)
N. Senthilkumar, V. Vijayakumar, S. Sarveswari, G.A. Gayathri, M. Gayathri, Iran J. Sci. Technol. Trans. Sci. 43, 735 (2019)
N. Kumar, S.R. Devineni, G. Singh, A. Kadirappa, S.K. Dubeya, P. Kumar, J. Pharm. Biomed. Anal. 119, 114 (2016)
B. Ahren, M. Landin-Olsson, P.A. Jansson, M. Svensson, D. Holmes, A. Schweizer, J. Clin. Endocrinol. Metab. 89, 2078 (2004)
R. Mentlein, B. Gallwitz, W.E. Schmidt, Eur. J. Biochem. 214, 829 (1993)
F. Shiri, M. Teymoori, Med. Chem. Res. 26, 947 (2017)
M. Kontoyianni, L.M. Mantzanidou, D.L. Hadjipavlou, J. Med. Chem. 47, 558 (2004)
H.M. Berman, J. Westbrook, Z. Feng, G. Gilliand, T.N. Bhat, H. Weissig, I.N. Shindyaloy, P.E. Bourne, Nucleic Acids Res. 28, 235 (2000)
Z. Li, H. Wan, Y. Shi, P. Ouyang, J. Chem. Inf. Comput. Sci. 44, 1886 (2004)
M.J. Abraham, T. Murtola, R. Schulz, S. Pall, J.C. Smith, B. Hess, E. Lindahl, SoftwareX 1–2, 19 (2015)
S.K. Singh, N. Manne, M. Pal, Beilstein J. Org. Chem. 4, 1 (2008)
A. Srivastava, R.M. Singh, Indian J. Chem. 44B, 1868 (2005)
D.C. Mungra, M.P. Patel, D.P. Rajani, R.G. Patel, Eur. J. Med. Chem. 46, 4192 (2011)
H. Fukushima, A. Hiratate, M. Takahashi, A. Mikami, M. Saito-Hori, E. Munetomo, K. Kitano, S. Chonan, H. Saito, A. Suzuki, Y. Takaoka, K. Yamamoto, Bioorg. Med. Chem. 16, 4093 (2008)
Acknowledgements
Authors are thankful to the administration, VIT University, Vellore, India, for providing facilities to carry out research work and also thankful to SIF-Chemistry for providing NMR facility. Authors are thankful to IISc Bangalore and IIT Madras for HRMS facility. Author P. Hemanth Kumar is thankful to the VIT University for providing Research Associateship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hemanth Kumar, P., Jyothish Kumar, L., Pavithrra, G. et al. Design, synthesis and exploration of in silico α-amylase and α-glucosidase binding studies of pyrrolidine-appended quinoline-constrained compounds. Res Chem Intermed 46, 1869–1880 (2020). https://doi.org/10.1007/s11164-019-04068-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04068-9