Abstract
Purpose
Pituitary adenomas are common CNS tumors that can cause endocrine dysfunction due to hormone oversecretion and by mass effect on the normal gland. The study of pituitary adenomas and adjacent sellar anatomy with high-resolution 7 T MRI may further characterize endocrine dysfunction. The purpose of this study was to determine the efficacy of 7 T MRI in identifying radiological markers for endocrine function.
Methods
MR images obtained in 23 patients with pituitary adenomas were reviewed by consensus between three neuroradiologists. Landmarks and criteria were devised to measure radiological features of stalk, tumor, and normal gland. Fischer’s exact tests and nominal logistic regression were performed.
Results
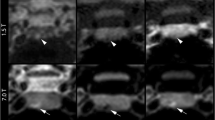
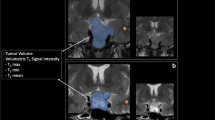
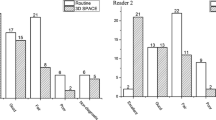
Mean cross-sectional area of the stalk just below the infundibular recess was 6.3 ± 3.7 mm2. Mean curvature and deviation angles were 34.2° ± 23.2° and 29.7° ± 17.3°, respectively. Knosp scores obtained differed between 7 T and lower field strength scans (P < 0.0001 [right] and P = 0.0006 [left]). Ability to characterize tumor was rated higher at 7 T compared with lower field MRI, P = 0.05. Confidence in visualizing normal gland was also higher using 7 T MRI, P = 0.036. The six hormone-secreting tumors had higher corrected T2 mean SI than non-secreting tumors (2.54 vs. − 0.38, P = 0.0196). Seven patients had preoperative hypopituitarism and had significantly greater stalk curvature angles than patients without hypopituitarism (71.7° vs. 36.55°, P = 0.027).
Conclusion
Radiological characterization of pituitary adenomas and adjacent native pituitary tissue may benefit with the use of 7 T MRI. Corrected T2 SI of tumor may be a sensitive predictor of hormonal secretion and may be useful in the diagnostic work-up for pituitary adenoma.
Summary statement
7 T MRI may be valuable in identifying markers of endocrine function in patients with pituitary adenomas. Our results indicate that hormone-secreting tumors have higher T2-weighted SI and tumors associated with preoperative hypopituitarism have greater stalk curvature angles.






Similar content being viewed by others
Abbreviations
- ROI :
-
Region of interest
- SI:
-
Signal intensity
- SNR:
-
Signal-to-noise ratio
- T :
-
Tesla
References
Satogami N, Miki Y, Koyama T, Kataoka M, Togashi K (2010) Normal pituitary stalk: high-resolution MR imaging at 3T. AJNR Am J Neuroradiol 31(2):355–359. https://doi.org/10.3174/ajnr.A1836
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87(3):933–963. https://doi.org/10.1152/physrev.00006.2006
Yuh WT, Fisher DJ, Nguyen HD, Tali ET, Gao F, Simonson TM, Schlechte JA (1994) Sequential MR enhancement pattern in normal pituitary gland and in pituitary adenoma. AJNR Am J Neuroradiol 15(1):101–108
Ahmadi J, North CM, Segall HD, Zee CS, Weiss MH (1986) Cavernous sinus invasion by pituitary adenomas. AJR Am J Roentgenol 146(2):257–262. https://doi.org/10.2214/ajr.146.2.257
Burlacu MC, Maiter D, Duprez T, Delgrange E (2019) T2-weighted magnetic resonance imaging characterization of prolactinomas and association with their response to dopamine agonists. Endocrine 63(2):323–331. https://doi.org/10.1007/s12020-018-1765-3
Gutch M, Kumar S, Razi SM, Saran S, Gupta KK (2014) Pituitary stalk interruption syndrome: case report of three cases with review of literature. J Pediatr Neurosci 9(2):188–191. https://doi.org/10.4103/1817-1745.139363
Just N (2014) Improving tumour heterogeneity MRI assessment with histograms. Br J Cancer 111:2205–2213. https://doi.org/10.1038/bjc.2014.512
Kreutz J, Vroonen L, Cattin F, Petrossians P, Thiry A, Rostomyan L, Tshibanda L, Beckers A, Bonneville J-FJN (2015) Intensity of prolactinoma on T2-weighted magnetic resonance imaging: towards another gender difference 57 (7):679–684. https://doi.org/10.1007/s00234-015-1519-3
Smith MV, Laws ER Jr (1994) Magnetic resonance imaging measurements of pituitary stalk compression and deviation in patients with nonprolactin-secreting intrasellar and parasellar tumors: lack of correlation with serum prolactin levels. Neurosurgery 34(5):834–839 discussion 839
Simmons GE, Suchnicki JE, Rak KM, Damiano TR (1992) MR imaging of the pituitary stalk: size, shape, and enhancement pattern. AJR Am J Roentgenol 159(2):375–377. https://doi.org/10.2214/ajr.159.2.1632360
Peyster RG, Hoover ED, Adler LP (1984) CT of the normal pituitary stalk. AJNR Am J Neuroradiol 5(1):45–47
Sbardella E, Joseph RN, Jafar-Mohammadi B, Isidori AM, Cudlip S, Grossman AB (2016) Pituitary stalk thickening: the role of an innovative MRI imaging analysis which may assist in determining clinical management. Eur J Endocrinol 175(4):255–263. https://doi.org/10.1530/eje-16-0455
Saeki N, Hoshi S, Sunada S, Sunami K, Murai H, Kubota M, Tatsuno I, Iuchi T, Yamaura A (2002) Correlation of high signal intensity of the pituitary stalk in macroadenoma and postoperative diabetes insipidus. AJNR Am J Neuroradiol 23(5):822–827
Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R (2010) MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 49(2):1271–1281. https://doi.org/10.1016/j.neuroimage.2009.10.002
Tomayko MM, Reynolds CPJCC (1989) Determination of subcutaneous tumor size in athymic (nude) mice. Pharmacology 24(3):148–154. https://doi.org/10.1007/bf00300234
Balchandani P, Naidich TP (2015) Ultra-high-field MR neuroimaging. AJNR Am J Neuroradiol 36(7):1204–1215. https://doi.org/10.3174/ajnr.A4180
Kale SC, Chen XJ, Henkelman RM (2009) Trading off SNR and resolution in MR images. NMR Biomed 22(5):488–494. https://doi.org/10.1002/nbm.1359
Little AS, Kelly DF, White WL, Gardner PA, Fernandez-Miranda JC, Chicoine MR, Barkhoudarian G, Chandler JP, Prevedello DM, Liebelt BD, Sfondouris J, Mayberg MR (2019) Results of a prospective multicenter controlled study comparing surgical outcomes of microscopic versus fully endoscopic transsphenoidal surgery for nonfunctioning pituitary adenomas: the Transsphenoidal extent of resection (TRANSSPHER) Study.1. https://doi.org/10.3171/2018.11.Jns181238
Hofstetter CP, Mannaa RH, Mubita L, Anand VK, Kennedy JW, Dehdashti AR, Schwartz TH (2010) Endoscopic endonasal transsphenoidal surgery for growth hormone–secreting pituitary adenomas. 29 (4):E6. https://doi.org/10.3171/2010.7.Focus10173
Fahlbusch R, Buchfelder M (1988) Transsphenoidal surgery of parasellar pituitary adenomas. Acta Neurochir 92(1–4):93–99
Cottier JP, Destrieux C, Brunereau L, Bertrand P, Moreau L, Jan M, Herbreteau D (2000) Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology 215(2):463–469. https://doi.org/10.1148/radiology.215.2.r00ap18463
Mooney MA, Hardesty DA, Sheehy JP, Bird R, Chapple K, White WL, Little AS (2017) Interrater and intrarater reliability of the Knosp scale for pituitary adenoma grading. 126 (5):1714. https://doi.org/10.3171/2016.3.Jns153044
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4):610–617 discussion 617-618
Shen M, Zhang Q, Liu W, Wang M, Zhu J, Ma Z, He W, Li S, Shou X, Li Y, Zhang Z, Ye H, He M, Lu B, Yao Z, Lu Y, Qiao N, Ye Z, Zhang Y, Yang Y, Zhao Y, Wang YJN (2016) Predictive value of T2 relative signal intensity for response to somatostatin analogs in newly diagnosed acromegaly 58 (11):1057–1065. https://doi.org/10.1007/s00234-016-1728-4
Heck A, Emblem KE, Casar-Borota O, Bollerslev J, Ringstad G (2016) Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine 52(2):333–343. https://doi.org/10.1007/s12020-015-0766-8
Xu Z, Sheehan JP, Lee Vance M, Schlesinger D (2012) Hypopituitarism after stereotactic radiosurgery for pituitary adenomas. Neurosurgery 72(4):630–637. https://doi.org/10.1227/NEU.0b013e3182846e44%Jneurosurgery
Arafah BM, Prunty D, Ybarra J, Hlavin ML, Selman WR (2000) The dominant role of increased intrasellar pressure in the pathogenesis of hypopituitarism, hyperprolactinemia, and headaches in patients with pituitary adenomas. J Clin Endocrinol Metab 85(5):1789–1793. https://doi.org/10.1210/jcem.85.5.6611
Arafah BM (2002) Medical management of hypopituitarism in patients with pituitary adenomas. Pituitary 5(2):109–117
Bland (1990) Endocrine function in the stalk compression syndrome. Neuroendocrinology 52
Dula AN, Virostko J, Shellock FG (2014) Assessment of MRI issues at 7 T for 28 implants and other objects. AJR Am J Roentgenol 202(2):401–405. https://doi.org/10.2214/ajr.13.10777
Van de Moortele PF, Akgun C, Adriany G, Moeller S, Ritter J, Collins CM, Smith MB, Vaughan JT, Ugurbil K (2005) B(1) destructive interferences and spatial phase patterns at 7 T with a head transceiver array coil. Magn Reson Med 54(6):1503–1518. https://doi.org/10.1002/mrm.20708
Funding
The following funding was received for this study: NIH R01 CA202911 and the Icahn School of Medicine Capital Campaign, Translational and Molecular Imaging Institute, and Department of Radiology, Icahn School of Medicine at Mount Sinai. No other sources of funding were received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
PB (the Principal Investigator in this study) is a named inventor on patents relating to magnetic resonance imaging (MRI) and RF pulse design. The patents have been licensed to GE Healthcare, Siemens AG, and Philips International and PB receives royalty payments relating to these patents. PB is a named inventor on patents relating to Slice-selective adiabatic magnetization T2-preparation (SAMPA) for efficient T2-weighted imaging at ultrahigh field strengths, methods for producing a semi-adiabatic spectral-spatial spectroscopic imaging sequence and devices thereof, and semi-adiabatic spectral-spatial spectroscopic imaging. These patents have been filed through MSIP; they remain unlicensed, there is no discussion to license them in the near future, and there are consequently no royalties revolving around them. The remaining authors declare no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
-7 T MRI yielded Knosp scores that were significantly different than those derived using lower field strength imaging.
-Secreting tumors had higher average T2-weighted SI than non-secreting tumors.
-Tumors associated with preoperative hypopituitarism had greater stalk curvature angles.
Rights and permissions
About this article
Cite this article
Rutland, J.W., Pawha, P., Belani, P. et al. Tumor T2 signal intensity and stalk angulation correlates with endocrine status in pituitary adenoma patients: a quantitative 7 tesla MRI study. Neuroradiology 62, 473–482 (2020). https://doi.org/10.1007/s00234-019-02352-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02352-4