Abstract
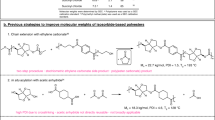
Practical and efficient method has been developed for the preparation of novel sulfur-containing esters from triglycerides as potential important industrial and biomaterials. The fact that these unusual compounds are not found in natural sources encourages both academic and industrial communities for their preparation with suitable chemical or enzymatic processes. In general, enzymatic processes requiring more laborious synthesis and product isolation stages. On the other hand, known chemical methods for the preparation of normal wax esters have several drawbacks cited in the present work. Therefore, the chemical method developed in the present study is environmentally benign and suitable for both small- and large-scale syntheses of normal and unusual wax esters. For this purpose, triglycerides were taken to the transesterification reaction in a solvent-free medium with synthetic thia-long-chain alcohols at a ratio of (1:3). In order to catalyze the reaction, newly synthesized bis-imidazole-based metal-free acidic ionic liquid was used and the thia-mono esters were obtained in a fairly short period of time (6 h) with good to excellent yields. The catalyst reuse and large-scale synthesis studies were also carried out.
Graphic abstract














Similar content being viewed by others
References
H.M. Alvarez, in Handbook of Hydrocarbon and Lipid Microbiology, ed. By K.N. Timmis (in-chief), T.J. McGenity, J.R. Van der Meer, V. De Lorenzo (Springer, Berlin, 2010) p. 2995
B.W. Darvell, Materials Science for Dentistry, 9th edn. (Woodhead Publishing, Cambridge, 2009), p. 390
P. Wu, H.J. Grav, R. Horn, J. Bremer, Biochem. Pharmacol. 51, 751 (1996)
M.S.F.L.K. Jie, M.S.K.S. Rahmatullah, J. Am. Oil Chem. Soc. 72, 1381 (1995)
S. Skrede, H.N. Sørensen, L.N. Larsen, H.H. Steineger, K. Høvik, Ø.S. Spydevold, R. Horn, J. Bremer, Biochim. Biophys. Acta, Lipids Lipid Metab. 1344 115 (1997)
M. Nerantzaki, K.V. Adam, I. Koliakou, E. Skoufa, A. Avgeropoulos, G.Z. Papageorgiou, D. Bikiaris, Macromol. Chem. Phys. 218, 1700305 (2017)
M. Desroches, S. Caillol, V. Lapinte, R. Auvergne, B. Boutevin, Macromolecules 44, 2489 (2011)
J. Skorve, A.C. Rustan, R.K. Berge, Lipids 30, 987 (1995)
D.K. Asiedu, L. Frøyland, H. Vaagenes, Ø. Lie, A. Demoz, R.K. Berge, Biochim. Biophys. Acta, Lipids Lipid Metab. 1300, 86 (1996)
K.J. Tronstad, Ø. Bruserud, K. Berge, R.K. Berge, Leukemia 16, 2292 (2002)
M.K. Pandey, A. Bansal, T.R. DeGrado, Heart Metab. 51, 15 (2011)
A. Aarsland, N. Aarsaether, J. Bremer, R.K. Berge, J. Lipid Res. 30, 1711 (1989)
H. Wang, X. Liu, Y. Wang, Y. Chen, Q. Jin, J. Ji, J. Mater. Chem. B 3, 3297 (2015)
U.S. Government. Code of Federal Regulations, vol. 3 Part 182, Section 182.3280, Code No. 21CFR182.3280 (2004)
A.O. Patil, S. Bodige, M.P. Hagemeister, U.S. Patent 0275129A1 (2015)
T. Noda, A. Nagata, Y. Abe, T. Sunada, JP Patent 2017081833A (2017)
M. Dexter, D. Steinberg, U.S. Patent 3758549A (1973)
J.M. Herdan, G. Valeanu, A. Popescu, J. Synth. Lubr. 12, 91 (1995)
K. Weissermel, H.D. Hermann, C. Heuck, C. Kuellmar, O. Mauz, M. Reiber, J. Winter, DE Patent 1117868 (1961)
H.Z. Lecher, N.J. Plainfield, H. Braus, U.S. Patent 3,222,318 (1965)
G. Michels, U. Jansen, F. Eisentrager, S. Kaminsky, DE Patent WO2017/211783A1 (2017)
W. Hiroaki, M. Harada, K. Nose, JP Patent WO2012049814A1 (2012)
A.S. Touchy, K. Kon, W. Onodera, K. Shimizu, Adv. Synth. Catal. 357, 1499 (2015)
K. Ishihara, M. Nakayama, S. Ohara, H. Yamamoto, Tetrahedron 5, 8179 (2002)
A. Sakthivel, K. Komura, Y. Sugi, Ind. Eng. Chem. Res. 47, 2538 (2008)
F. Liu, K. Huang, S. Ding, S. Dai, J. Mater. Chem. A 4, 14567 (2016)
Q. Wu, F. Liu, X. Yi, Y. Zoud, L. Jiang, Green Chem. 20, 1020 (2018)
F. Liu, K. Huang, Q. Wu, S. Dai, Adv. Mater. 29, 1700445 (2017)
F. Liu, K. Huang, A. Zheng, F.S. Xiao, S. Dai, ACS Catal. 8, 372 (2018)
F. Liu, C. Liu, W. Kong, C. Qi, A. Zheng, S. Dai, Green Chem. 18, 6536 (2016)
N. Weber, E. Klein, K. Vosmann, J. Agric. Food Chem. 54, 2957 (2003)
N. Ieda, K. Mantri, Y. Miyata, A. Ozaki, K. Komura, Y. Sugi, Ind. Eng. Chem. Res. 47, 8631 (2008)
H. Chen, X. Xu, L. Liu, G. Tang, Y. Zhao, RSC Adv. 3, 16247 (2013)
K. Mantri, K. Komura, Y. Sugi, Synthesis 12, 1939 (2005)
A.C. Cole, J.L. Jenson, I. Ntai, K.L.T. Tran, K.J. Wearver, D.C. Forbes, J.H. Davis, J. Am. Chem. Soc. 124, 5962 (2002)
H.P. Zhu, F. Yang, J. Tang, M.Y. He, Green Chem. 5, 38 (2003)
J. Gui, X. Cong, D. Liu, X. Zhang, Z. Hu, Z. Sun, Catal. Commun. 5, 473 (2004)
H. Zhang, F. Xu, X. Zhou, G. Zhang, C. Wang, Green Chem. 9, 1208 (2007)
O. Kocian, K. Stransky, J. Zavada, Collect. Czechoslov. Chem. Commun. 47, 1356 (1982)
B. Boutevin, A. El Idrissi, J.P. Parkisi, Makromol. Chem. 191, 445 (1990)
M. Mansueto, C.K. Kreß, S. Laschat, Tetrahedron 70, 6258 (2014)
J. Kirres, K. Schmitt, I. Wurzbach, F. Giesselmann, S. Ludwigs, M. Ringenberg, A. Ruff, A. Baro, S. Laschat, Org. Chem. Front. 4, 790 (2017)
J.F. Xu, Y.Z. Chen, L.Z. Wu, C.H. Tung, Q.Z. Yang, Org. Lett. 15, 6148 (2013)
R. Sheldon, Chem. Commun. 0, 2399 (2001)
A.S. Amarasekara, Chem. Rev. 116, 6133 (2016)
A. Yıldırım, S. Mudaber, S. Öztürk, Eur. J. Lipid Sci. Technol. 121, 1800303 (2019)
A. Yıldırım, K. Kıraylar, Turk. J. Chem. 43, 802 (2019)
J.L. Anderson, R. Ding, A. Ellern, D.W. Armstrong, J. Am. Chem. Soc. 127, 593 (2005)
H. Eshghi, M. Rahimizadeh, M. Hasanpour, M. Bakavoli, Res. Chem. Intermed. 41, 4187 (2015)
MarvinSketch (Product Version: 15.6.29.0, calculation module developed by ChemAxon) (2015)
S.Y. Bae, M.D. Winemiller, J. Org. Chem. 78, 6457 (2013)
L.M. Ling, X.H. Ling, S. Ming, Y. Ling, H.S. Tong, Phosphorus. Sulfur Silicon Relat. Elem. 189, 387 (2014)
Y.C. Yang, L.L. Szafraniec, W.T. Beaudry, J.R. Ward, J. Org. Chem. 53, 3293 (1988)
P. Sun, S. Liu, Y. Zhou, S. Zhang, Z. Yao, ACS Sustainable Chem. Eng. 6, 13579 (2018)
B.J.K. Ahn, S. Kraft, X.S. Sun, J. Agric. Food Chem. 60, 2179 (2012)
Acknowledgements
This work was financially supported by the Bursa Uludağ University Scientific Research Projects Unit (OUAP(F)-2018/3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yıldırım, A., Kıraylar, K. & Öztürk, S. A convenient approach directly from triglycerides toward the producing of thia-wax esters as bio- and chemical raw materials. Res Chem Intermed 46, 215–230 (2020). https://doi.org/10.1007/s11164-019-03944-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03944-8