Abstract
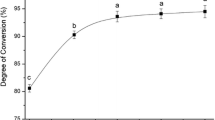
The time course study of high monoester mixtures from soybean oil (HMMS) synthesis, as healthier alternatives to trans food products, in a supercritical CO2 (SCCO2) medium with and without enzyme, was investigated. Phosphorous nuclear magnetic resonance (31P-NMR) was used to quantify the absolute amount of partially esterified acylglycerols (PEGs). Carbon NMR was utilized to determine the type and position of the fatty acids (FAs) of HMMS. Enzyme and time significantly influenced the synthesis of 1-monoglycerides (1-MGs), 2-MGs, and 1,2-diglycerides (1,2-DGs) in this alcoholysis of soybean oil with 1,2-propanediol, based on high catalytic activity and operational stability of Novozym 435 in SCCO2 during short reaction time. Results suggest that 4 h is a suitable reaction time for this lipase-catalyzed interesterification (LIE) system for the synthesis of 2-MGs with a yield of 20%. The highest polyunsaturated fatty acid (PUFA) (65%) in the triglyceride (TG) of HMMS was produced after 4 h of reaction. After 6 h of reaction, a high level (20%) of saturated fatty acids (SFAs) was found in the TGs of HMMS, which were distributed between the sn-2 (5%) and sn-1, 3 (15%) positions.






Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- CIE:
-
Chemical interesterification
- CTDP:
-
2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane
- CVD:
-
Cardiovascular disease
- DGs:
-
Diglycerides
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FAs:
-
Fatty acids
- FDA:
-
Food and Drugs Administration-USA
- GRAS:
-
Generally recognized as safe
- HMMS:
-
High monoester mixtures from soybean oil
- LIE:
-
Lipase-catalyzed interesterification
- MGs:
-
Monoglycerides
- MUFAs:
-
Monounsaturated fatty acids
- PEGs:
-
Partially esterified acylglycerols
- 31P-NMR :
-
Phosphorous nuclear magnetic resonance
- PUFA:
-
Polyunsaturated fatty acid
- SCCO2 :
-
Supercritical CO2
- SFAs:
-
Saturated fatty acids
- SLs:
-
Structured lipids
- TG:
-
Triglyceride
- UFAs:
-
Unsaturated fatty acids
References
Wang, D. D., & Hu, F. B. (2017). Dietary fat and risk of cardiovascular disease: recent controversies and advances. Annual Review of Nutrition, 37(1), 423–446. https://doi.org/10.1146/annurev-nutr-071816-064614.
VHM & PP Study Group, Ulmer H, Borena W, et al (2009) Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. British Journal of Cancer 101:1202–1206. https://doi.org/10.1038/sj.bjc.6605264, 7.
Handelsman, Y., & Shapiro, M. D. (2017). Triglycerides, atherosclerosis, and cardiovascular outcome studies: focus on. Endocrine Practice : Official Journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists, 23(1), 100–112. https://doi.org/10.4158/EP161445.RA.
Kadhum, A. A. H., & Shamma, M. N. (2017). Edible lipids modification processes: a review. Critical Reviews in Food Science and Nutrition, 57(1), 48–58. https://doi.org/10.1080/10408398.2013.848834.
Martin, D., Moran-Valero, M. I., Vázquez, L., Reglero, G., & Torres, C. F. (2014). Comparative in vitro intestinal digestion of 1,3-diglyceride and 1-monoglyceride rich oils and their mixtures. Food Research International, 64, 603–609. https://doi.org/10.1016/j.foodres.2014.07.026.
Feltes, M. M. C., de Oliveira, D., Block, J. M., & Ninow, J. L. (2013). The production, benefits, and applications of monoacylglycerols and diacylglycerols of nutritional interest. Food and Bioprocess Technology, 6(1), 17–35. https://doi.org/10.1007/s11947-012-0836-3.
Pfeffer, J., Freund, A., Bel-Rhlid, R., Hansen, C.-E., Reuss, M., Schmid, R. D., & Maurer, S. C. (2007). Highly efficient enzymatic synthesis of 2-monoacylglycerides and structured lipids and their production on a technical scale. Lipids, 42(10), 947–953. https://doi.org/10.1007/s11745-007-3084-y.
Zhang, Y., Wang, X., Zou, S., Xie, D., Jin, Q., & Wang, X. (2018). Synthesis of 2-docosahexaenoylglycerol by enzymatic ethanolysis. Bioresource Technology, 251, 334–340. https://doi.org/10.1016/j.biortech.2017.12.025.
List, G. R. (2014). Trans fats replacement solutions for frying and baking applications, shortenings, margarines, and spreads. In Trans Fats Replacement Solutions (pp. 245–273). Elsevier. doi:https://doi.org/10.1016/B978-0-9830791-5-6.50016-2.
Klonoff, D. C. (2007). Replacements for trans fats—will there be an oil shortage? Journal of Diabetes Science and Technology, 1(3), 415–422. https://doi.org/10.1177/193229680700100316.
Orthoefer, F. (2008). Applications of emulsifiers in baked foods. In G. L. Hasenhuettl & R. W. Hartel (Eds.), Food emulsifiers and their applications (pp. 263–284). New York: Springer New York. https://doi.org/10.1007/978-0-387-75284-6_9.
Doucet, J. 2005. Shortening composition. US20050214436A1.
Szeląg, H., & Sadecka, E. (2009). Influence of sodium dodecyl sulfate presence on esterification of propylene glycol with lauric acid. Industrial & Engineering Chemistry Research, 48(18), 8313–8319. https://doi.org/10.1021/ie8019449.
Damstrup, M. L., Jensen, T., Sparsø, F. V., Kiil, S. Z., Jensen, A. D., & Xu, X. (2006). Production of heat-sensitive monoacylglycerols by enzymatic glycerolysis in tert-pentanol: process optimization by response surface methodology. Journal of the American Oil Chemists’ Society, 83(1), 27–33. https://doi.org/10.1007/s11746-006-1171-5.
Pawongrat, R., Xu, X., & H-Kittikun, A. (2007). Synthesis of monoacylglycerol rich in polyunsaturated fatty acids from tuna oil with immobilized lipase AK. Food Chemistry, 104(1), 251–258. https://doi.org/10.1016/j.foodchem.2006.11.036.
Lee, L. Y., Chin, N. L., Christensen, E. S., Lim, C. H., Yusof, Y. A., & Talib, R. A. (2018). Applications and effects of monoglycerides on frozen dessert stability. LWT, 97, 508–515. https://doi.org/10.1016/j.lwt.2018.07.020.
Remonatto, D., Santin, C. M. T., Valério, A., Lerin, L., Batistella, L., Ninow, J. L., et al. (2015). Lipase-catalyzed glycerolysis of soybean and canola oils in a free organic solvent system assisted by ultrasound. Applied Biochemistry and Biotechnology, 176(3), 850–862. https://doi.org/10.1007/s12010-015-1615-1.
Zhen, Z., Ma, X., Huang, H., Li, G., & Wang, Y. (2017a). Enzymatic production of highly unsaturated monoacyglycerols and diacylglycerols and their emulsifying effects on the storage stability of a palm oil based shortening system. Journal of the American Oil Chemists’ Society, 94(9), 1175–1188. https://doi.org/10.1007/s11746-017-3023-x.
Damstrup, M. L. (2008). Proces development of enzymatic glycerolysis for industrial monoacylglycerol production. Technical University of Denmark, Lyngby, Denmark.
Li, X., Liu, P., Khan, F. I., Li, D., Yang, B., & Wang, Y. (2017). Substrate selectivity and optimization of immobilized SMG1-F278N lipase in synthesis of propylene glycol monooleate: SMG1-F278N lipase from Malassezia globosa. European Journal of Lipid Science and Technology, 119(5), 1600423. https://doi.org/10.1002/ejlt.201600423.
Acevedo, N. C., Block, J. M., & Marangoni, A. G. (2012). Unsaturated emulsifier-mediated modification of the mechanical strength and oil binding capacity of a model edible fat crystallized under shear. Langmuir : the ACS journal of surfaces and colloids, 28(46), 16207–16217. https://doi.org/10.1021/la303365d.
Skogerson, L., & Boutté, T. (2010). Non-hydrogenated vegetable oil based shortening containing an elevated diglyceride emulsifier composition. KS: Lenexa.
Fureby, A. M., Adlercreutz, P., & Mattiasson, B. (1996). Glyceride synthesis in a solvent-free system. Journal of the American Oil Chemists’ Society, 73(11), 1489–1495. https://doi.org/10.1007/BF02523515.
Mensink, R. P., Sanders, T. A., Baer, D. J., Hayes, K., Howles, P. N., & Marangoni, A. (2016). The increasing use of interesterified lipids in the food supply and their effects on health parameters. Advances in Nutrition: An International Review Journal, 7(4), 719–729. https://doi.org/10.3945/an.115.009662.
Fiametti, K. G., Sychoski, M. M., Cesaro, A. D., Furigo, A., Bretanha, L. C., Pereira, C. M. P., et al. (2011). Ultrasound irradiation promoted efficient solvent-free lipase-catalyzed production of mono- and diacylglycerols from olive oil. Ultrasonics Sonochemistry, 18(5), 981–987. https://doi.org/10.1016/j.ultsonch.2010.11.010.
Willis, W. M., Lencki, R. W., & Marangoni, A. G. (1998). Lipid modification strategies in the production of nutritionally functional fats and oils. Critical Reviews in Food Science and Nutrition, 38(8), 639–674. https://doi.org/10.1080/10408699891274336.
Solaesa, Á. G., Sanz, M. T., Falkeborg, M., Beltrán, S., & Guo, Z. (2016). Production and concentration of monoacylglycerols rich in omega-3 polyunsaturated fatty acids by enzymatic glycerolysis and molecular distillation. Food Chemistry, 190, 960–967. https://doi.org/10.1016/j.foodchem.2015.06.061.
Jackson, M. A. (1998). Monoglyceride production via enzymatic glycerolysis of oils in supercritical CO2. (Morton, IL). Retrieved from http://www.freepatentsonline.com/5747305.html
Jackson, M. A., & King, J. W. (1997). Lipase-catalyzed glycerolysis of soybean oil in supercritical carbon dioxide. Journal of the American Oil Chemists’ Society, 74(2), 103–106. https://doi.org/10.1007/s11746-997-0152-7.
Liu, K.-J., & Shaw, J.-F. (1995). Synthesis of propylene glycol monoesters of docosahexaenoic acid and eicosapentaenoic acid by lipase-catalyzed esterification in organic solvents. Journal of the American Oil Chemists’ Society, 72(11), 1271–1274. https://doi.org/10.1007/BF02546198.
Temelli, F., King, J. W., & List, G. R. (1996). Conversion of oils to monoglycerides by glycerolysis in supercritical carbon dioxide media. Journal of the American Oil Chemists’ Society, 73(6), 699–706. https://doi.org/10.1007/BF02517943.
Akoh, C. C. (2017). Food lipids: chemistry, nutrition, and biotechnology, Fourth Edition. CRC Press. https://doi.org/10.1201/9781315151854.
Shaw, J.-F., & Lo, S. (1994). Production of propylene glycol fatty acid monoesters by lipase-catalyzed reactions in organic solvents. Journal of the American Oil Chemists’ Society, 71(7), 715–719. https://doi.org/10.1007/BF02541427.
Ibrahim, N. A. B. (2007). Structured triacylglycerol of palm-based margarine fat by enzymatic interesterification. PhD Thesis, Technical University of Denmark.
Srivastava, S., Madras, G., & Modak, J. (2003). Esterification of myristic acid in supercritical carbon dioxide. The Journal of Supercritical Fluids, 27(1), 55–64.
Vieira, S. A., McClements, D. J., & Decker, E. A. (2015). Challenges of utilizing healthy fats in foods. Advances in Nutrition, 6(3), 309S–317S. https://doi.org/10.3945/an.114.006965.
Irimescu, R., Iwasaki, Y., & Hou, C. T. (2002). Study of TAG ethanolysis to 2-MAG by immobilized Candida antarctica lipase and synthesis of symmetrically structured TAG. Journal of the American Oil Chemists’ Society, 79(9), 879–883. https://doi.org/10.1007/s11746-002-0573-8.
Anderson, E. M., Larsson, K. M., & Kirk, O. (1998). One biocatalyst–many applications: the use of Candida antarctica B-lipase in organic synthesis. Biocatalysis and Biotransformation, 16(3), 181–204. https://doi.org/10.3109/10242429809003198.
King, J. W. (2004). Critical fluid technology for the processing of lipid-related natural products. Comptes Rendus Chimie, 7(6), 647–659. https://doi.org/10.1016/j.crci.2004.02.008.
Tai, H. P., & Brunner, G. (2011). Mono- and di-acylglycerol synthesis in CO2-expanded acetone. The Journal of Supercritical Fluids, 59, 87–91. https://doi.org/10.1016/j.supflu.2011.07.021.
King, J. (2005). Supercritical fluid processing of nutritionally functional lipids. In Healthful lipids. AOCS Publishing. Retrieved from http://www.crcnetbase.com/doi/abs/10.1201/9781439822289.ch6
Fregolente, P. B. L., Fregolente, L. V., Pinto, G. M. F., Batistella, B. C., Wolf-Maciel, M. R., & Filho, R. M. (2008). Monoglycerides and diglycerides synthesis in a solvent-free system by lipase-catalyzed glycerolysis. Applied Biochemistry and Biotechnology, 146(1–3), 165–172. https://doi.org/10.1007/s12010-008-8133-3.
Weber, N., & Mukherjee, K. D. (2004). Solvent-free lipase-catalyzed preparation of diacylglycerols. Journal of Agricultural and Food Chemistry, 52(17), 5347–5353. https://doi.org/10.1021/jf0400819.
Silveira, A., Erick, S. M.-P., Basso, A., Serban, S., Mamede, R. P., Tardioli, P. W., Farinas, C. S., Rocha-Martin, J., Fernandez-Lorente, G., & Guisan, J. M. (2017). Modulation of the regioselectivity of Thermomyces Lanuginosus lipase via biocatalyst engineering for the ethanolysis of oil in fully anhydrous medium. BMC Biotechnology, 17(1), 88. https://doi.org/10.1186/s12896-017-0407-9.
Gunstone, F. D. (1999). Enzymes as biocatalysts in the modification of natural lipids. Journal of the Science of Food and Agriculture, 79(12), 1535–1549. https://doi.org/10.1002/(SICI)1097-0010(199909)79:12<1535::AID-JSFA430>3.0.CO;2-7.
Shimotoyodome, A., Osaki, N., Onizawa, K., Mizuno, T., Suzukamo, C., Okahara, F., et al. (2012). Dietary 1-monoolein decreases postprandial GIP release by reducing jejunal transport of glucose and fatty acid in rodents. American Journal of Physiology-Gastrointestinal and Liver Physiology, 303(3), G298–G310. https://doi.org/10.1152/ajpgi.00457.2011.
Lubary, M. (2011). Added-value milk fat derivatives from integrated processes using supercritical technology. Universitat de Barcelona, Spain.
Gouk, S. W., Cheng, S. F., Ong, A. S. H., & Chuah, C. H. (2011). Rapid and direct quantitative analysis of positional fatty acids in triacylglycerols using 13C NMR. European Journal of Lipid Science and Technology, 114(5), 510–519. https://doi.org/10.1002/ejlt.201100074.
Compton, D. L., Eller, F. J., Laszlo, J. A., & Evans, K. O. (2012). Purification of 2-monoacylglycerols using liquid CO2 extraction. Journal of the American Oil Chemists’ Society., 89(8), 1529–1536. https://doi.org/10.1007/s11746-012-2035-9.
Vlahov, G. (2009). 13C nuclear magnetic resonance spectroscopy to determine fatty acid distribution in triacylglycerols of vegetable oils with “high - low oleic acid” and “high linolenic acid.” The Open Magnetic Resonance Journal, 2(1), 8–19. https://doi.org/10.2174/1874769800902010008.
Funding
This study was supported by the Manitoba Pulse and Soybean Growers (MPSG), The Natural Sciences and Engineering Research Council of Canada (NSERC), and Mitacs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vafaei, N., Eskin, M.N.A., Rempel, C.B. et al. Interesterification of Soybean Oil with Propylene Glycol in Supercritical Carbon Dioxide and Analysis by NMR Spectroscopy. Appl Biochem Biotechnol 191, 905–920 (2020). https://doi.org/10.1007/s12010-019-03200-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03200-0