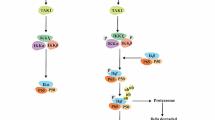
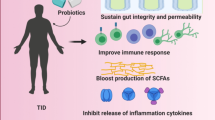
Abstract
Diabetes and other lifestyle disorders have been recognized as the leading cause of morbidity and mortality globally. Nuclear factor kappa B (NF-κB) is a major factor involved in the early pathobiology of diabetes and studies reveal that hyperglycemic conditions in body leads to NF-κB mediated activation of several cytokines, chemokines and inflammatory molecules. NF-κB family comprises of certain DNA-binding protein factors that elicit the transcription of pro-inflammatory molecules. Various studies have identified NF-κB as a promising target for diabetic management. Probiotics have been proposed as bio-therapeutic agents for treatment of inflammatory disorders and many other chronic clinical stages. The precise mechanisms by which probiotics acts is yet to be fully understood, however research findings have indicated their role in NF-κB modulation. The current review highlights NF-κB as a bio-therapeutic target for probable management of type 2 diabetes through probiotic intervention.


Similar content being viewed by others
Abbreviations
- NF-κB:
-
Nuclear factor kappa B
- LAB:
-
Lactic acid bacteria
- IDF:
-
International diabetes federation
References
Cho N, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B (2018) IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Panwar H, Rashmi HM, Batish VK, Grover S, (2013) Probiotics as potential biotherapeutics in the management of type 2 diabetes–prospects and perspectives. Diabetes Metab Res Rev 29(2):103–112
Salsali A, Nathan M (2006) A review of types 1 and 2 diabetes mellitus and their treatment with insulin. Am J Ther 13(4):349–361
Ndisang JF, Vannacci A, Rastogi S (2017) Insulin resistance, type 1 and type 2 diabetes, and related complications. J Diabetes Res. https://doi.org/10.1155/2017/1478294
Jayachandran M, Vinayagam R, Ambati RR, Xu B, Chung SSM, (2018) Guava leaf extract diminishes hyperglycemia and oxidative stress, prevents β-cell death, inhibits inflammation, and regulates NF-κB signaling pathway in STZ induced diabetic rats. BioMed Res Int. https://doi.org/10.1155/2018/4601649
Lyssenko V, Laakso M (2013) Genetic screening for the risk of type 2 diabetes: worthless or valuable? Diabetes Care 36:S120–S126
Baeyens L, Lemper M, Staels W, De Groef S, De Leu N, Heremans Y et al (2018) (Re) generating human beta cells: status, pitfalls, and perspectives. Physiol Rev 98(3):1143–1167
Kulkarni RN (2004) The islet β-cell. Int J Biochem Cell Biol 36(3):365–371
Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL, (2005) Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(suppl 2):S97–S107
Nogueira TC, Paula FM, Villate O, Colli ML, Moura RF, Cunha DA et al (2013) GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic beta cell apoptosis via regulation of a splice variant of the BH3-only protein Bim. PLoS Genet. https://doi.org/10.1371/journal.pgen.1003532
Ardestani A, Paroni F, Azizi Z, Kaur S, Khobragade V, Yuan T et al (2014) MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat Med 20(4):385
Coto E, Díaz-Corte C, Tranche S, Gómez J, Alonso B, Iglesias S et al (2018) Gene variants in the NF-KB pathway (NFKB1, NFKBIA, NFKBIZ) and their association with type 2 diabetes and impaired renal function. Hum Immunol 79(6):494–498
Chen H, Zhou W, Ruan Y, Yang L, Xu N, Chen R et al (2018) Reversal of angiotensin ll-induced β-cell dedifferentiation via inhibition of NF-κb signaling. Mol Med 24(1):43
Guinane CM, Cotter PD (2013) Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol 6(4):295–308
Gomes AC, Bueno AA, de Souza RGM, Mota JF (2014) Gut microbiota, probiotics and diabetes. Nutr J 13(1):60
Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK, (2011) Understanding the role of gut microbiome–host metabolic signal disruption in health and disease. Trends Microbiol 19(7):349–359
Elshaghabee F, Rokana N, Panwar H, Heller KJ, Schrezenmeir J (2019) Probiotics for dietary management of non-alcoholic fatty liver disease. Environ Chem Lett. https://doi.org/10.1007/s10311-019-00896-8
Thakur N, Rokana N, Panwar H (2016) Probiotics: selection criteria, safety and role in health and disease. J Innov Biol 3(1):259–270
Floch MH, Montrose DC (2005) Use of probiotics in humans: an analysis of the literature. Gastroenterol Clin 34(3):547–570
Panwar H, Calderwood D, Grant IR, Grover S, Green BD (2014) Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur J Nutr 53(7):1465–1474
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC (2014) Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506
Llewellyn A, Foey A (2017) Probiotic modulation of innate cell pathogen sensing and signalling events. Nutrients 9(10):1156
Yan F, Polk DB (2010) Probiotics: progress toward novel therapies for intestinal diseases. Curr Opin Gastroenterol 26(2):95
Ghosh S, May MJ, Kopp EB (1998) NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Ann Rev Immunol 16(1):225–260
Pahl HL (1999) Activators and target genes of Rel/NF-κB transcription factors. Oncogene. https://doi.org/10.1038/sj.onc.1203239
Tang C, Zhu G (2019) Classic and novel signaling pathways involved cancer: targeting the NF-κB and Syk signaling pathways. Curr Stem Cell Res Ther 14(3):219–225
Li Q, Verma IM (2002) NF-κB regulation in the immune system. Nat Rev Immunol 2(10):725
Senftleben U, Karin M (2002) The Ikk/nf-κb pathway. Crit Care Med 30(1):S18–S26
Silverman N, Maniatis T (2001) NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev 15(18):2321–2342
Chen ZJ, Parent L, Maniatis T (1996) Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell 84(6):853–862
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-κB activity. Ann Rev Immunol 18(1):621–663
Zhou LZH, Johnson AP, Rando TA (2001) NFκB and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med 31(11):1405–1416
Serasanambati M, Chilakapati SR (2016) Function of nuclear factor kappa B (NF-kB) in human diseases-a review. South Indian J Biol Sci 2(4):368–387
Patel S, Santani D (2009) Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol Rep 61(4):595–603
Arakelyan A, Nersisyan L, Poghosyan D, Khondkaryan L, Hakobyan A, Löffler-Wirth H et al (2017) Autoimmunity and autoinflammation: a systems view on signaling pathway dysregulation profiles. PLoS ONE. https://doi.org/10.1371/journal.pone.0187572
Eizirik DL, Mandrup-Poulsen T (2001) A choice of death–the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44(12):2115–2133
Cardozo AK, Heimberg H, Heremans Y, Leeman R, Kutlu B, Kruhøffer M et al (2001) A comprehensive analysis of cytokine-induced and nuclear factor-κB-dependent genes in primary rat pancreatic β-cells. J Biol Chem 276(52):48879–48886
Ding L, Fan L, Xu X, Fu J, Xue Y (2019) Identification of core genes and pathways in type 2 diabetes mellitus by bioinformatics analysis. Mol Med Rep 20:2597–2608
Li L, Pan Z, Yang S, Shan W, Yang Y (2018) Identification of key gene pathways and coexpression networks of islets in human type 2 diabetes. Diabetes Metab Syndr Obes 11:553–563
Luo B, Huang F, Liu Y, Liang Y, Wei Z, Ke H, Zeng Z, Huang W, He Y (2017) NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. Front physiol 8:519
Zhang N, Valentine JM, Zhou Y, Li ME, Zhang Y, Bhattacharya A, Walsh ME, Fisher KE, Austad SN, Osmulski P, Gaczynska M, Shoelson SE, Remmen HV, Chen HI, Chen Y, Liang H, Musi N (2017) Sustained NFkB inhibition improves insulin sensitivity but is detrimental to muscle health. Aging Cell 16:847–858
Giannoukakis N, Mi Z, Rudert WA, Gambotto A, Trucco M, Robbins P (2000) Prevention of beta cell dysfunction and apoptosis activation in human islets by adenoviral gene transfer of the insulin-like growth factor I. Gene Thera 7(23):2015
Ho E, Quan N, Tsai YH, Lai W, Bray TM (2001) Dietary zinc supplementation inhibits NFκB activation and protects against chemically induced diabetes in CD1 mice. Exp Biol Med 226(2):103–111
Mabley JG, Hasko G, Liaudet L, Soriano F, Southan GJ, Salzman AL et al (2002) NFkappaB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocin-induced diabetes. J Endocrinol 173(3):457–464
Lamhamedi-Cherradi SE, Zheng S, Hilliard BA, Xu L, Sun J, Alsheadat S et al (2003) Transcriptional regulation of type I diabetes by NF-κB. J Immunol 171(9):4886–4892
Kumar D, Robertson S, Burns KD (2004) Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem 259(1–2):67–70
Song MY, Jeong GS, Kwon KB, Ka SO, Jang HY, Park JW et al (2010) Sulfuretin protects against cytokine-induced beta-cell damage and prevents streptozotocin-induced diabetes. Exp Mol Med 42(9):628–638
Collier JJ, Burke SJ, Eisenhauer ME, Lu D, Sapp RC, Frydman CJ et al (2011) Pancreatic β-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS ONE. https://doi.org/10.1371/journal.pone.0022485
Burke SJ, Lu D, Sparer TE, Masi T, Goff MR, Karlstad MD et al (2013) NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am J Physiol Endocrinol Metab 306(2):E131–E149
Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM et al (2005) IKK-β links inflammation to obesity-induced insulin resistance. Nat Med 11(2):191
Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J et al (2005) Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11(2):183
Hayden MS, Ghosh S (2014) Regulation of NF-kB by TNF family cytokines. Semin Immunol 26(3):253–266
Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 95(5):2409–2415
Gautam A, Gupta S, Mehndiratta M, Sharma M, Singh K, Kalra OP, Agarwal S, Gambhir JK (2017) Association of NFKB1 gene polymorphism (rs28362491) with levels of inflammatory biomarkers and susceptibility to diabetic nephropathy in Asian Indians. World J Diabetes 8(2):66
Romzova M, Hohenadel D, Kolostova K, Pinterova D, Fojtikova M, Ruzickova S, Dostal C, Bosak V, Rychlik I, Cerna M (2006) NFκB and its inhibitor IκB in relation to type 2 diabetes and its microvascular and atherosclerotic complications. Hum Immunol 67(9):706–713
Cernea S, Dobreanu M (2013) Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med 23(3):266–280
Norlin S, Ahlgren U, Edlund H (2005) Nuclear factor-κB activity in β-cells is required for glucose-stimulated insulin secretion. Diabetes 54(1):125–132
Liuwantara D, Elliot M, Smith MW, Yam AO, Walters SN, Marino E, McShea A, Grey ST (2006) Nuclear factor- κB regulates β cell death. Diabetes 55(9):2491–2501
Madsen KL (2012) Enhancement of epithelial barrier function by probiotics. J Epithel Biol Pharmacol 5(1):55–59
Hole AS, Grimmer S, Naterstad K, Jensen MR, Paur I, Johansen SG, Balstad TR, Blomhoff R, Sahlstrom S (2009) Activation and inhibition of nuclear factor kappa B activity by cereal extracts: role of dietary phenolic acids. J Agric Food Chem 57:9481–9488
Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B, Yan W, Tang T (2014) Plumbagin inhibits LPS-induced inflammation through the inactivation of the nuclear factor-kappa B and mitogen activated protein kinase signaling pathways in RAW 264.7 cells. Food Chem Toxicol 64:177–183
Huang CS, Fan YE, Lin CY, Hu ML (2007) Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J Nutr Biochem 18(7):449–456
Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjanheikki J, Koistinaho J (2004) Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem 91(3):755–765
Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppée JY et al (2006) Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol 176(2):1228–1237
Ma D, Forsythe P, Bienenstock J (2004) Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression. Infect Immun 72(9):5308–5314
Zhang L, Li N, Caicedo R, Neu J (2005) Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α–induced interleukin-8 production in caco-2 cells. J Nutr 135(7):1752–1756
O'hara AM, O'regan P, Fanning Á, O'mahony C, MacSharry J, Lyons A, et al (2006) Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology 118(2):202–215
Bu X, Lian X, Wang Y, Luo C, Tao S, Liao Y, Yang J, Chen A, Yang Y (2019) Dietary yeast culture modulates immune response related to TLR2-MyD88-NF-kβ signaling pathway, antioxidant capability and disease resistance against Aeromonas hydrophila for Ussuri catfish (Pseudobagrus ussuriensis). Fish Shellfish Immunol 84:711–718
Sun KY, Xu DH, Xie C, Plummer S, Tang J, Yang XF et al (2017) Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine 92:1–11
Kaci G, Lakhdari O, Doré J, Ehrlich SD, Renault P, Blottière HM et al (2011) Inhibition of the NF-κB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl Environ Microbiol 77(13):4681–4684
Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S et al (2008) The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76(9):4163–4175
Kim SW, Kim HM, Yang KM, Kim SA, Kim SK, An MJ et al (2010) Bifidobacterium lactis inhibits NF-κB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm Bowel Dis 16(9):1514–1525
Lakhdari O, Tap J, Béguet-Crespel F, Le Roux K, De Wouters T, Cultrone A et al (2011) Identification of NF-κB modulation capabilities within human intestinal commensal bacteria. BioMed Res Int. https://doi.org/10.1155/2011/282356
Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ et al (2009) Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Rad Biol Med 47(8):1205–1211
Johnson-Henry KC, Nadjafi M, Avitzur Y, Mitchell DJ, Ngan BY, Galindo-Mata E et al (2005) Amelioration of the effects of Citrobacter rodentium infection in mice by pretreatment with probiotics. J Infect Dis 191(12):2106–2117
Breyner NM, Michon C, de Sousa CS, Vilas Boas PB, Chain F, Azevedo VA, Langella P, Chatel JM (2017) Microbial anti-inflammatory molecule (MAM) from Faecalibacterium prausnitzii shows a protective effect on DNBS and DSS-induced colitis model in mice through inhibition of NF-κB pathway. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00114
Lee SK, Yang KM, Cheon JH, Kim TI, Kim WH (2012) Anti-inflammatory mechanism of Lactobacillus rhamnosus GG in lipopolysaccharide-stimulated HT-29 cell. Korean J Gastroenterol 60(2):86–93
Wagner RD, Johnson SJ (2017) Probiotic bacteria prevent Salmonella–induced suppression of lymphoproliferation in mice by an immunomodulatory mechanism. BMC Microbiol 17(1):77
Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG et al (2004) Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 5:104–112
Voltan S, Martines D, Elli M, Brun P, Longo S, Porzionato A et al (2008) Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-γ in the intestinal mucosa. Gastroenterol 135(4):1216–1227
Volpe C, Villar-Delfino PH, Dos Anjos P, Nogueira-Machado JA (2018) Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis 9(2):119
Kumar A, Wu H, Collier-Hyams LS, Hansen JM, Li T, Yamoah K et al (2007) Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J 26(21):4457–4466
Donato KA, Gareau MG, Wang YJJ, Sherman PM (2010) Lactobacillus rhamnosus GG attenuates interferon-γ and tumour necrosis factor-α-induced barrier dysfunction and pro-inflammatory signalling. Microbiol 156(11):3288–3297
Liu Y, Fatheree NY, Mangalat N, Rhoads JM (2012) Lactobacillus reuteri strains reduce incidence and severity of experimental necrotizing enterocolitis via modulation of TLR4 and NFκB signaling in the intestine. Am J Physiol Heart Circ Physiol 302(6):G608–G617
Iyer C, Kosters A, Sethi G, Kunnumakkara AB, Aggarwal BB, Versalovic J, (2008) Probiotic Lactobacillus reuteri promotes TNF-induced apoptosis in human myeloid leukemia-derived cells by modulation of NF-κB and MAPK signalling. Cell Microbiol 10(7):1442–1452
Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M (2004) Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 53(6):821–828
Nishitani Y, Tanoue T, Yamada K, Ishida T, Yoshida M, Azuma T et al (2009) Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int Immunopharmacol 9(12):1444–1451
Versalovic J, Iyer C, Ping Lin Y, Huang Y, Dobrogosz W (2008) Commensal-derived probiotics as anti-inflammatory agents. Microbial Ecol Health Dis 20(2):86–93
Rahman MM, McFadden G (2011) Modulation of NF-κB signalling by microbial pathogens. Nat Rev Microbiol 9(4):291
Lee JM, Hwang KT, Jun WJ, Park CS, Lee MY (2008) Antiinflammatory effect of lactic acid bacteria: inhibition of cyclooxygenase-2 by suppressing nuclear factor-kappaB in Raw 264.7 macrophage cells. J Microbiol Biotechnol 18(10):1683–1688
Petrof EO, Claud EC, Sun J, Abramova T, Guo Y, Waypa TS et al (2009) Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflamm Bowel Dis 15(10):1537–1547
Ma X, Hua J, Li Z (2008) Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol 49:821–830
Jang SE, Hyam SR, Han MJ, Kim SY, Lee BG, Kim DH (2013) Lactobacillus brevis G-101 ameliorates colitis in mice by inhibiting NF-κB, MAPK and AKT pathways and by polarizing M1 macrophages to M2-like macrophages. J Appl Microbiol 115(3):888–896
Eom JS, Song J, Choi HS (2015) Protective effects of a novel probiotic strain of Lactobacillus plantarum JSA22 from traditional fermented soybean food against infection by Salmonella enterica serovar Typhimurium. J Microbiol Biotechnol 25(4):479–491
Sougioultzis S, Simeonidis S, Bhaskar KR, Chen X, Anton PM, Keates S et al (2006) Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem Biophys Res Commun 343(1):69–76
Zhang M, Jin X, Yang YF (2019) β-Glucan from Saccharomyces cerevisiae induces SBD-1 production in ovine ruminal epithelial cells via the dectin-1–Syk–NF-κB signaling pathway. Cell Signal 53:304–315
Le TK, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB, Pham TP, Tran QB, Le TH, Da Pham X (2015) Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res 36(1):63–70
O'Mahony C, Scully P, O'Mahony D, Murphy S, O'Brien F, Lyons A et al (2008) Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-κB activation. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1000112
Khan S, Maremanda KP, Jena G. (2019) Butyrate, a short-chain fatty acid and histone deacetylases inhibitor: nutritional, physiological, and pharmacological aspects in diabetes. In: Handbook of nutrition, diet, and epigenetics Cham: Springer. 793–807
Kumar A, Wu H, Collier-Hyams LS, Kwon YM, Hanson JM, Neish AS, (2009) The bacterial fermentation product butyrate influences epithelial signaling via reactive oxygen species-mediated changes in cullin-1 neddylation. J Immunol 182(1):538–546
Toumi R, Soufli I, Rafa H, Belkhelfa M, Biad A, Touil-Boukoffa C (2014) Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int J Immunopathol Pharmacol 27(4):615–627
Maciel FR, Punaro GR, Rodrigues AM, Bogsan CS, Rogero MM, Oliveira MN, Mouro MG, Higa EM (2016) Immunomodulation and nitric oxide restoration by a probiotic and its activity in gut and peritoneal macrophages in diabetic rats. Clin Nutr 35(5):1066–1072
Bernini LJ, Simão ANC, de Souza CH, Alfieri DF, Segura LG, Costa GN, Dichi I (2018) Effect of Bifidobacterium lactis HN019 on inflammatory markers and oxidative stress in subjects with and without the metabolic syndrome. Br J Nutr 120(6):645–652
Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, Corneli RB, Ferretti E, Gulino A, Grasso F, De Simone C (2005) Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia 48(8):1565–1575
Lavasani S, Dzhambazov B, Nouri M, Fåk F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Weström B (2010) A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE. https://doi.org/10.1371/journal.pone.0009009
Garidou L, Pomie C, Klopp P, Waget A, Charpentier J, Aloulou M, Giry A, Serino M, Stenman L, Lahtinen S, Dray C, Iacovoni JS, Courtney M, Collet X, Amar J, Servant F, Lelouvier B, Valet P, Eberl G, Fazilleau N, Douin-Echinard V, Heymes C, Burcelin R (2015) The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease. Cell Metab 22(1):100–112
Topol I, Kamyshny A (2013) Study of expression of TLR2, TLR4 and transckription factor NF-kB structures of galt of rats in the conditions of the chronic social stress and modulation of structure of intestinal microflora. Georgian Med News 12(225):115–120
Ganesan K, Chung SK, Vanamala J, Xu B (2018) Causal relationship between diet-induced gut microbiota changes and diabetes: a novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int J Mol 19(12):3720
Bagarolli RA, Tobar N, Oliveira AG, Araújo TG, Carvalho BM, Rocha GZ, Vecina JF, Calisto K, Guadagnini D, Prada PO, Santos A (2017) Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J Nutr Biochem 50:16–25
Trapecar M, Goropevsek A, Gorenjak M, Gradisnik L, Rupnik MS (2014) A co-culture model of the developing small intestine offers new insight in the early immunomodulation of enterocytes and macrophages by Lactobacillus spp. through STAT1 and NF-kB p65 Translocation. PLoS ONE. https://doi.org/10.1371/journal.pone.0086297
Li Z, Yang S, Lin H, Huang J, Watkins PA, Moser AB et al (2003) Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatol 37(2):343–435
Reichold A, Brenner SA, Spruss A, Förster-Fromme K, Bergheim I, Bischoff SC (2014) Bifidobacterium adolescentis protects from the development of nonalcoholic steatohepatitis in a mouse model. J Nutr Biochem 25(2):118–125
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhardwaj, R., Singh, B.P., Sandhu, N. et al. Probiotic mediated NF-κB regulation for prospective management of type 2 diabetes. Mol Biol Rep 47, 2301–2313 (2020). https://doi.org/10.1007/s11033-020-05254-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05254-4