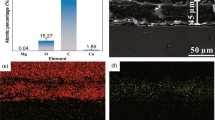
Abstract
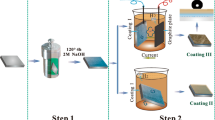
A hydrophobic surface was successfully fabricated on the Mg-Al-layered double hydroxide (Mg-Al LDH)/Mg(OH)2-coated AZ31 magnesium alloy via an in-situ steam coating (SC) process and a subsequent surface modification with environment-friendly myristic acid (MA). The microstructure, composition and hydrophobicity of SC/MA composite coating were investigated by XRD, SEM, EDS, FTIR, and contact angle (CA) measurement. The corrosion behavior of the hybrid coating was evaluated by potentiodynamic polarization, EIS and hydrogen evolution test in 3.5 wt.% NaCl solution. The results showed that the LDH coating had nano-flake microstructure, which remained unchanged after modification with MA. The CA of the MA-modified coating surface reached up to 129°± 3.5°, and the corrosion current density of SC/MA-2 coating decreased about three orders of the magnitude compared to that of the substrate. It is proven that the modified surface has an effective anti-corrosion effect on AZ31 alloy. The formation mechanism and the corrosion mechanism of the coating were also discussed.
Similar content being viewed by others
References
Jia P, Wu M, Zhang J, et al. Effects of Mg-Zn-Y quasicrystal addition on the microstructures, mechanical performances and corrosion behaviors of as-cast AM60 magnesium alloy. Materials Research Express, 2018, 5(10): 106512
Pan F S, Zeng B, Jiang B, et al. Enhanced mechanical properties of AZ31B magnesium alloy thin sheets processed by on-line heating rolling. Journal of Alloys and Compounds, 2017, 693: 414–120
Li F, Liu Y, Li X B. Dynamic recrystallization behavior of AZ31 magnesium alloy processed by alternate forward extrusion. Frontiers of Materials Science, 2017, 11(3): 296–305
Jin T, Wang Y, Yin H, et al. Corrosion protection properties and mechanism of epoxy/acetic acid-doped polyaniline coating on magnesium alloy. Journal of Nanoscience and Nanotechnology, 2018, 18(7): 4992–5000
Jin H, Yang X, Wang M. Chemical conversion coating on AZ31B magnesium alloy and its corrosion tendency. Acta Metallurgica Sinica (English Letters), 2009, 22(1): 65–70
Ishizaki T, Kudo R, Omi T, et al. Magnesium hydroxide/magnesium phosphate compounds composite coating for corrosion protection of magnesium alloy by a combination process of chemical conversion and steam curing. Materials Letters, 2012, 68: 122–125
Zhang F, Zhang C L, Song L, et al. Corrosion resistance of superhydrophobic Mg-Al layered double hydroxide coatings on aluminum alloys. Acta Metallurgica Sinica (English Letters), 2015, 28(11): 1373–1381
Li C Y, Fan X L, Zeng R C, et al. Corrosion resistance of in-situ growth of nano-sized Mg(OH)2 on micro-arc oxidized magnesium alloy AZ31 - Influence of EDTA. Journal of Materials Science and Technology, 2019, 35(6): 1088–1098
Singh B P, Jena B K, Bhattacharjee S, et al. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surface and Coatings Technology, 2013, 232: 475–481
Zeng R C, Qi W C, Song Y W, et al. In vitro degradation of MAO/PLA coating on Mg-1.21Li-1.12Ca-1.0Y alloy. Frontiers of Materials Science, 2014, 8(4): 343–353
Jin T, Han Y, Bai R, et al. Corrosion protection properties of nano NH2-reduced graphene oxide/epoxy composite coatings formed by self-curing on magnesium alloy. Journal of Nanoscience and Nanotechnology, 2018, 18(7): 4971–4981
Liang J, Zhang R H, Peng Z J, et al. One-step electrochemical fabrication of bilayered MgO/polymer coating on magnesium alloy. Frontiers of Materials Science, 2014, 8(3): 307–312
Jin T, Kong F M, Bai R Q, et al. Anti-corrosion mechanism of epoxy-resin and different content Fe2O3 coatings on magnesium alloy. Frontiers of Materials Science, 2016, 10(4): 367–374
Li L Y, Cui L Y, Zeng R C, et al. Advances in functionalized polymer coatings on biodegradable magnesium alloys - A review. Acta Biomaterialia, 2018, 79: 23–36
Zhang G, Wu L, Tang A T, et al. Growth behavior of MgAl-layered double hydroxide films by conversion of anodic films on magnesium alloy AZ31 and their corrosion protection. Applied Surface Science, 2018, 456: 419–429
Wu L, Yang D N, Zhang G, et al. Fabrication and characterization of Mg-M layered double hydroxide films on anodized magnesium alloy AZ31. Applied Surface Science, 2018, 431: 177–186
Zhang G, Wu L, Tang A T, et al. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corrosion Science, 2018, 139: 370–382
Ishizaki T, Kamiyama N, Watanabe K, et al. Corrosion resistance of Mg(OH)2/Mg-Al layered double hydroxide composite film formed directly on combustion-resistant magnesium alloy AMCa602 by steam coating. Corrosion Science, 2015, 92: 76–84
Guo L, Zhang F, Lu J C, et al. A comparison of corrosion inhibition of magnesium aluminum and zinc aluminum vanadate intercalated layered double hydroxides on magnesium alloys. Frontiers of Materials Science, 2018, 12(2): 198–206
Chen J, Song Y W, Shan D Y, et al. Study of the in situ growth mechanism of Mg-Al hydrotalcite conversion film on AZ31 magnesium alloy. Corrosion Science, 2012, 63: 148–158
Chen J, Song Y, Shan D, et al. Study of the corrosion mechanism of the in situ grown Mg-Al-CO32− hydrotalcite film on AZ31 alloy. Corrosion Science, 2012, 65: 268–277
Zeng R C, Li X T, Liu Z G, et al. Corrosion resistance of Zn-Al layered double hydroxide/poly(lactic acid) composite coating on magnesium alloy AZ31. Frontiers of Materials Science, 2015, 9(4): 355–365
Li D D, Wang F Y, Yu X, et al. Anticorrosion organic coating with layered double hydroxide loaded with corrosion inhibitor of tungstate. Progress in Organic Coatings, 2011, 71(3): 302–309
Zeng R C, Liu Z G, Zhang F, et al. Corrosion of molybdate intercalated hydrotalcite coating on AZ31 Mg alloy. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2014, 2(32): 13049–13057
Yao Q S, Zhang F, Song L, et al. Corrosion resistance of a ceria/polymethyltrimethoxysilane modified Mg-Al-layered double hydroxide on AZ31 magnesium alloy. Journal of Alloys and Compounds, 2018, 764: 913–928
Kamiyama N, Panomsuwan G, Yamamoto E, et al. Effect of treatment time in the Mg(OH)2/Mg-Al LDH composite film formed on Mg alloy AZ31 by steam coating on the corrosion resistance. Surface and Coatings Technology, 2016, 286: 172–177
Ishizaki T, Chiba S, Suzuki H. In situ formation of anticorrosive Mg-Al layered double hydroxide-containing magnesium hydroxide film on magnesium alloy by steam coating. ECS Electrochemistry Letters, 2013, 2(5): C15–C17
Ishizaki T, Chiba S, Watanabe K, et al. Corrosion resistance of Mg-Al layered double hydroxide container-containing magnesium hydroxide films formed directly on magnesium alloy by chemical-free steam coating. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2013, 1(31): 8968–8977
Huang S, Peng H, Tjiu W W, et al. Assembling exfoliated layered double hydroxide (LDH) nanosheet/carbon nanotube (CNT) hybrids via electrostatic force and fabricating nylon nanocomposites. The Journal of Physical Chemistry B, 2010, 114(50): 16766–16772
Liao H, Jia Y, Wang L, et al. Size effect of layered double hydroxide platelets on the crystallization behavior of isotactic polypropylene. ACS Omega, 2017, 2(8): 4253–4260
Zhang J, Gu C, Tu J. Robust slippery coating with superior corrosion resistance and anti-icing performance for AZ31B Mg alloy protection. ACS Applied Materials & Interfaces, 2017, 9(12): 11247–11257
Yuan Z, Wang X, Bin J, et al. Controllable fabrication of lotus-leaf-like superhydrophobic surface on copper foil by self-assembly. Applied Physics A: Materials Science & Processing, 2014, 116(4): 1613–1620
Richard E, Aruna S T, Basu B J. Superhydrophobic surfaces fabricated by surface modification of alumina particles. Applied Surface Science, 2012, 258(24): 10199–10204
Cao N, Miao Y Y, Zhang D L, et al. Preparation of mussel-inspired perfluorinated polydopamine film on brass substrates: Superhydrophobic and anti-corrosion application. Progress in Organic Coatings, 2018, 125: 109–118
Kuang J, Ba Z, Li Z, et al. Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surface and Coatings Technology, 2019, 361: 75–82
Zhang G, Tang A T, Wu L, et al. In-situ grown super- or hydrophobic Mg-Al layered double hydroxides films on the anodized magnesium alloy to improve corrosion properties. Surface and Coatings Technology, 2019, 366: 238–247
Zhao L, Liu Q, Gao R, et al. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corrosion Science, 2014, 80: 177–183
Ding P, Li Z Z, Wang Q, et al. In situ growth of layered double hydroxide films under dynamic processes: Influence of metal cations. Materials Letters, 2012, 77: 1–3
Ozturk S, Balkose D, Okur S, et al. Effect of humidity on electrical conductivity of zinc stearate nanofilms. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2007, 302(1–3): 67–74
Wang Y H, Wang W, Zhong L, et al. Super-hydrophobic surface on pure magnesium substrate by wet chemical method. Applied Surface Science, 2010, 256(12): 3837–3840
Xi J, Feng L, Jiang L. A general approach for fabrication of superhydrophobic and superamphiphobic surfaces. Applied Physics Letters, 2008, 92(5): 053102
Zhang F, Liu Z G, Zeng R C, et al. Corrosion resistance of Mg-Al-LDH coating on magnesium alloy AZ31. Surface and Coatings Technology, 2014, 258: 1152–1158
Zhang F, Zhang C L, Zeng R C, et al. Corrosion resistance of the superhydrophobic Mg(OH)2/Mg-Al layered double hydroxide coatings on magnesium alloys. Metals, 2016, 6(4): 85
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51601108, 21676285 and 51571134) and the Scientific Research Foundation of Shandong University of Science and Technology for Recruited Talents (2017RCJJ015).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qiu, ZM., Zhang, F., Chu, JT. et al. Corrosion resistance and hydrophobicity of myristic acid modified Mg-Al LDH/Mg(OH)2 steam coating on magnesium alloy AZ31. Front. Mater. Sci. 14, 96–107 (2020). https://doi.org/10.1007/s11706-020-0492-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-020-0492-x