Abstract
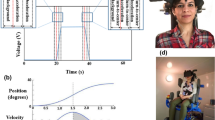
Electrovestibulography (EVestG), a technology purported to measure vestibular activity at the vestibular periphery, was used to compare the vestibular responses to two sensory inputs: (1) back-forward physical tilt (with eyes-open and eyes-closed) and (2) virtual reality replica of the back-forward tilt (eyes-open, physically static). Twenty-seven healthy participants (10 females) were tested. From each of the EVestG recordings, two feature curves: (1) average field potential (FP), and (2) distribution of time intervals between the detected FPs were extracted. For the eyes-closed physical tilt, except for the background segment, the FP response curve was generally wider compared to that evoked during the virtual replica tilt (p < 0.05). Moreover, the eyes-closed physical tilt produced longer time intervals between FP’s compared to the virtual stimulus. For this measure, for the background segment, the eyes closed and open physical tilt responses were significantly different (p < 0.05) in both ears (repeated measure experimental design). The results support: (1) both vestibular and visual inputs evoking a measurably different EVestG response, (2) the differences between physical and virtual vestibular responses are dependent on the eyes being either open or closed, and (3) for the stimuli used, the modulation of vestibular afferent activity was measurably smaller for virtual than physical stimulation.





Similar content being viewed by others
Abbreviations
- AP:
-
Action potential
- EEG:
-
Electroencephalography
- EMG:
-
Electromyography
- EOG:
-
Electrooculography
- EVestG:
-
Electrovestibulography
- EVS:
-
Efferent vestibular system
- FP:
-
Field potential
- IH33:
-
Interval histogram
- NEER:
-
Neural event extraction routine
- RTC:
-
Return-to-center
- VN:
-
Vestibular nuclei
References
Alahmari, K. A., P. J. Sparto, G. F. Marchetti, M. S. Redfern, J. M. Furman, and S. L. Whitney. Comparison of virtual reality based therapy with customized vestibular physical therapy for the treatment of vestibular disorders. IEEE Trans. Neural Syst. Rehabil. Eng. 22:389–399, 2013.
Azzena, G. B., O. Mameli, and E. Tolu. Distribution of visual input to the vestibular nuclei. Arch. Ital. Biol. 118:196–204, 1980.
Blakley, B., Z. A. Dastgheib, B. Lithgow, and Z. Moussavi. Preliminary report: neural firing patterns specific for Meniere’s disease. J. Otolaryngol. Head Neck Surg. 43:52, 2014.
Brandt, T., P. Bartenstein, A. Janek, and M. Dieterich. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 121:1749–1758, 1998.
Brown, D. J., and R. B. Patuzzi. Evidence that the compound action potential (CAP) from the auditory nerve is a stationary potential generated across dura mater. Hear. Res. 267:12–26, 2010.
Cullen, K. E. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 35:185–196, 2012.
Curthoys, I. S., J. W. Grant, A. M. Burgess, C. J. Pastras, D. J. Brown, and L. Manzari. Otolithic receptor mechanisms for vestibular-evoked myogenic potentials: a review. Front. Neurol. 9:366, 2018.
Geller, A. S., J. F. Burke, M. R. Sperling, A. D. Sharan, B. Litt, G. H. Baltuch, T. H. Lucas, II, and M. J. Kahana. Eye closure causes widespread low-frequency power increase and focal gamma attenuation in the human electrocorticogram. Clin. Neurophysiol. 125:1764, 2014.
Goldberg, J. M., and C. Fernández. Efferent vestibular system in the squirrel monkey: anatomical location and influence on afferent activity. J. Neurophysiol. 43:986–1025, 1980.
Heibert, D., and B. Lithgow. Modelling the vestibular head tilt response. Australas. Phys. Eng. Sci. Med. 28:37–42, 2005.
Jensen, O., and A. Mazaheri. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4:186, 2010.
Kolb, H. How the Retina Works: much of the construction of an image takes place in the retina itself through the use of specialized neural circuits. Am. Sci. 91:28–35, 2003.
Kolb, H., E. Fernandez, and R. Nelson. Visual Acuity–Webvision: the Organization of the Retina and Visual System. 1–26, 2012. http://www.ncbi.nlm.nih.gov/books/NBK11530/
Leijon, S., and A. K. Magnusson. Physiological characterization of vestibular efferent brainstem neurons using a transgenic mouse model. PLoS ONE 9:e98277, 2014.
Lithgow, B. A methodology for detecting field potentials from the external ear canal: NEER and EVestG. Ann. Biomed. Eng. 40(8):1835–1850, 2012. https://doi.org/10.1007/s10439-012-0526-3.
Lithgow, B. J., A. L. Garrett, Z. M. Moussavi, C. Gurvich, J. Kulkarni, J. J. Maller, and P. B. Fitzgerald. Major depression and electrovestibulography. World J. Biol. psychiatry 16(5):334–350, 2015.
Lithgow, B. J., and Z. Moussavi. Physiological differences in the follicular, luteal, and menstrual phases in healthy women determined by electrovestibulography: depression, anxiety, or other associations? Neuropsychobiology 76:72–81, 2017.
Lithgow, B. J., Z. Moussavi, and P. B. Fitzgerald. Quantitative separation of the depressive phase of bipolar disorder and major depressive disorder using electrovestibulography. World J. Biol. Psychiatry 2019. https://doi.org/10.1080/15622975.2019.1599143.
Lithgow, B. J., Z. Moussavi, C. Gurvich, J. Kulkarni, J. J. Maller, and P. B. Fitzgerald. Bipolar disorder in the balance. Eur. Arch. Psychiatry Clin. Neurosci. 2018. https://doi.org/10.1007/s00406-018-0935-x.
Longstaff, A. Instant notes in Neuroscience (BIOS Instant Notes). Abingdon: Taylor & Francis, 2005.
Manso, A. Vestibular rehabilitation with visual stimuli in peripheral vestibular disorders. Braz. J. Otorhinolaryngol. 82:232–241, 2016.
Mathews, M. A., A. J. Camp, and A. J. Murray. Corrigendum: reviewing the role of the efferent vestibular system in motor and vestibular circuits. Front. Physiol. 8:552, 2018. https://doi.org/10.3389/fphys.2017.00552.
Meldrum, D., S. Herdman, R. Moloney, D. Murray, D. Duffy, K. Malone, H. French, S. Hone, R. Conroy, and R. McConn-Walsh. Effectiveness of conventional versus virtual reality based vestibular rehabilitation in the treatment of dizziness, gait and balance impairment in adults with unilateral peripheral vestibular loss: a randomised controlled trial. BMC Ear Nose Throat Disord. 12:3, 2012.
Paige, G. D., L. Telford, S. H. Seidman, and G. R. Barnes. Human vestibuloocular reflex and its interactions with vision and fixation distance during linear and angular head movement. J. Neurophysiol. 80:2391–2404, 1998.
Pavlou, M., A. Lingeswaran, R. A. Davies, M. A. Gresty, and A. M. Bronstein. Simulator based rehabilitation in refractory dizziness. J. Neurol. 251:983–995, 2004.
Ryugo, D. K., R. R. Fay, and A. N. Popper. Auditory and Vestibular Efferents. New York: Springer, p. 545, 1995.
Sadeghi, S. G., J. M. Goldberg, L. B. Minor, and K. E. Cullen. Efferent-mediated responses in vestibular nerve afferents of the alert macaque. J. Neurophysiol. 101:988–1001, 2009.
Simes, R. J. An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751–754, 1986.
Suleiman, A., B. Lithgow, B. Mansouri, and Z. Moussavi. Investigating the validity and reliability of Electrovestibulography (EVestG) for detecting post-concussion syndrome (PCS) with and without comorbid depression. Sci. Rep. 8:14495, 2018.
White, B. J., J. Y. Kan, R. Levy, L. Itti, and D. P. Munoz. Superior colliculus encodes visual saliency before the primary visual cortex. Proc. Natl. Acad. Sci. USA. 114:9451–9456, 2017.
Zikovitz, D. C. Self-motion perception through visual optic flow and vestibular cues., 1998. https://www.collectionscanada.gc.ca/obj/s4/f2/dsk2/ftp01/MQ39249.pdf.
Acknowledgments
This study was partly supported by the Natural science and engineering research council (NSERC) of Canada.
Conflict of interest
All authors declare no conflicts of interest and disclose no affiliations with or involvement in any organization or entity that could potentially bias the subject matter or materials discussed in this manuscript. Figure 1c is the author (MA) who has agreed to its publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Tingrui Pan oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1
The Neural Pathways Connecting Visual and Vestibular Systems
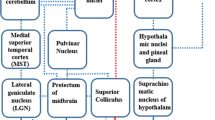
Photoreceptors in the eyes convert light information received from the surrounding environment to electrical signals. These electrical signals can pass along horizontal, bipolar and or amacrine cells to reach ganglion cells, wherein primary visual processing tasks place (e.g., contrast sensitivity, color perception). The axons of the ganglion cells form the optic nerves, which transmit visual information. The visual system and some of the involved regions have been depicted in the below figure. These regions include the medial superior temporal cortex (MST) and vestibulocerebellar areas (mainly involved in motion information processing), the posterior parietal cortex (responsible for the detection of shapes), and inferotemporal cortex (involved in color perception). These brain regions have connections to the vestibular nuclei, which in turn projects via the efferent vestibular system (pathway) to the peripheral vestibular system (semicircular canals and otolith organs (saccule and utricle)).

On the left: Schematic diagram of the human eye with some key structures labeled and the ‘wiring’ of cells in the human retina.12 Adapted from “How the Retina Works: Much of the construction of an image takes place in the retina itself through the use of specialized neural circuits”, by Helga Kolb, 2003, Am Sci 91 28–35. Adapted with permission. On the right: Visual projections towards the vestibular processing brain regions.
Appendix 2

Rights and permissions
About this article
Cite this article
Ashiri, M., Lithgow, B., Suleiman, A. et al. Differences Between Physical vs. Virtual Evoked Vestibular Responses. Ann Biomed Eng 48, 1241–1255 (2020). https://doi.org/10.1007/s10439-019-02446-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02446-3