Abstract
The present study reports the development of a novel defective TiO2−x catalyst with oxygen vacancy (OV) which carries dual types of reaction sites for H2O2 activation. The performance of this catalyst on the degradation of organic pollutants was evaluated using organic dyes [such as methyl orange, methylene blue, and rhodamine B (RhB)] as model pollutants. The defects in TiO2−x exhibited a wide pH working window (pH 2–9) for RhB degradation which is wider than that of traditional Fenton systems. Furthermore, this catalyst retained its high catalytic activity even after five cycles. It was confirmed that the surface OV and Ti3+ of TiO2−x served as the active sites for H2O2 activation while the oxygen vacancy promoted adsorption of the organic pollutants, thus enhancing the Fenton-like catalytic performance. The results are applicable to Fenton catalysts via surface engineering and can stimulate new opportunities for the optimization of defect-type Fenton catalysts.
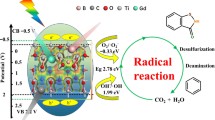
Graphic Abstract
This study, reports the defect of TiO2−x oxygen vacancy (OV) catalyst with oxygen vacancy (OV) which carries dual types of reaction sites for H2O2 activation. The performance of this catalyst on the degradation of organic pollutants was evaluated using organic dyes [such as methyl orange, methylene blue, and rhodamine B (RhB)] as model pollutants. The defects in TiO2−x exhibited a wide pH working window (pH 2–9) for RhB degradation which is wider than that of traditional Fenton systems. Figure shows the schematic of the reaction mechanism. First, H2O2 adsorbs on the TiO2−x surfaces; consequently, the OV and Ti3+ serve as the “Fenton-catalytic” center for H2O2 activation to produce ·OH radicals on the TiO2−x surface. Meanwhile, OV on TiO2−x surface is beneficial for the adsorbed organic pollutants. Because of the generated hydroxyl radical on the TiO2−x surface is very close to the adsorbed pollutant. As a result, the rapid reaction of in situ generated hydroxyl radical with the adsorbed organic pollutants on the TiO2−x surface giving rise to excellent Fenton-like catalytic performance. The proposed overall Fenton-like reaction mechanism on oxygen deficient TiO2−x catalyst.








Similar content being viewed by others
References
Kwan WP, Voelker BM (2003) Environ Sci Technol 37(6):1150
Pouran SR, Aziz ARA, Wan MAWD (2015) J Ind Eng Chem 21(1):53–69
Klavarioti M, Mantzavinos D, Kassinos D (2009) Environ Int 35(2):0–417
Bobu M, Yediler A, Siminiceanu I (2013) Environ Lett 48(3):251–262
Shokri A, Mahanpoor K, Soodbar D (2016) Desalin Water Treat 57(35):16473–16482
Wang Q, Tian S, Ning P (2014) Ind Eng Chem Res 53(15):6334–6340
Bokare AD, Choi W (2014) J Hazard Mater 275:121–135
Parida KM, Pradhan AC (2010) Ind Eng Chem Res 49(18):8310–8318
Kishimoto N, Kitamura T, Kato M et al (2013) Water Res 47(5):1919–1927
Costa RCC, Moura FCC, Ardisson JD et al (2008) Appl Catal B 83(1–2):131–139
Li J, Ai Z, Zhang L (2009) J Hazard Mater 164(1):18–25
Zhang S, Gao H, Huang Y et al (2018) Environ Sci 5(5):1179–1190
Liang H, Zhou S, Chen Y et al (2015) J Taiwan Inst Chem Eng 49:105–112
Hendriksen BLM, Ackermann MD, Van Rijn R et al (2010) Nat Chem 2(9):730
Martinez U, Vilhelmsen LB, Kristoffersen HH et al (2011) Phys Rev B 84(20):205434
Li H, Shang J, Zhu H et al (2016) ACS Catal 6(12):8276–8285
Zhuang J, Dai W, Tian Q et al (2010) Langmuir 26(12):9686–9694
Wang XH, Li JG, Kamiyama H et al (2006) J Phys Chem B 110(13):6804–6809
Li H, Shang J, Yang Z et al (2017) Environ Sci Technol 51(10):5685–5694
Jin H, Tian X, Nie Y et al (2017) Environ Sci Technol 51(21):12699–12706
An X, Tang Q, Lan H et al (2019) Appl Catal B 244:407–413
Lei F, Sun Y, Liu K et al (2014) J Am Chem Soc 136(19):6826–6829
Zhang N, Li X, Ye H et al (2016) J Am Chem Soc 138(28):8928–8935
Pan XY, Yang MQ, Fu XZ, Zhang N, Xu YJ (2013) Nanoscale 5:3601–3614
Wang GM, Ling YC, Li Y (2012) Nanoscale 4:6682–6691
Nowotny J (2008) Energy Environ Sci 1:565–572
Pei DN, Gong L, Zhang AY et al (2015) Nat Commun 6:8696
Zhou WY, Liu JY, Song JY et al (2017) Anal Chem 89(6):3386–3394
Liu G, Yang HG, Wang X et al (2009) J Phys Chem C 113(52):21784–21788
He D, Ma J, Collins RN et al (2016) Environ Sci Technol 50(7):3820–3828
Xia T, Zhang Y, Murowchick J et al (2014) Catal Today 225:2–9
Yu J, Zhao X, Du J et al (2000) J Sol-Gel Sci Technol 17(2):163–171
Lee HY, Wu BK, Chern MY (2014) Electron Mater Lett 10(1):51–55
Hou X, Huang X, Jia F et al (2017) Environ Sci Technol 51(9):5118–5126
Cahill AE, Taube H (1952) J Am Chem Soc 74(9):2312–2318
Orhanovic M, Earley JE (1975) Inorg Chem 14(7):1478–1481
Hao Y, Lai Q, Xu Z et al (2005) Solid State Ionics 176(13–14):1201–1206
Chen J, Li YF, Sit P et al (2013) J Am Chem Soc 135(50):18774–18777
Song Z, Wang B, Yu J et al (2017) Appl Surf Sci 413:292–301
Acknowledgements
The authors are thankful for the financial support from the National Natural Science Foundation of China (21273050, 21573048, 21873022, 51574092).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qiu, Jy., Chen, Jh., Xiao, By. et al. Oxygen Deficient TiO2−x with Dual Reaction Sites for Activation of H2O2 to Degrade Organic Pollutants. Catal Lett 150, 222–233 (2020). https://doi.org/10.1007/s10562-019-02920-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02920-6