Abstract
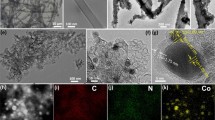
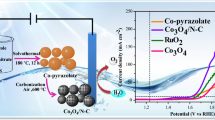
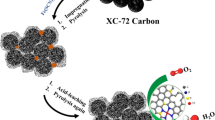
Herein, a novel cadmium nitrite initiated mesoporous carbon shell encapsulated binary cobalt/cobalt phosphide (Co/Co2P@MC-(Cdx)) composites were first prepared by a facile solvothermal reaction following a phosphorization procedure. The Co/Co2P@MC-(Cd0.5), with molar ratio of Cd/Co = 0.5 in the initial feeding stock, exhibited overpotentials of as low as 99 mV at 10 mA cm−2 and small Tafel slopes of 53 mV dec−1 under 0.5 M H2SO4 conditions. Various techniques further demonstrated that the superior activity of Co/Co2P@MC-(Cd0.5) was attributed to the introduction of Cd initiator, which resulted in the mesoporisity on carbon shell and the modified electron state between the Co and Co2P. This novel Cd initiated mesoporous carbon encapsulated Co/Co2P composites hold promising potential for utilization in the hydrogen evolution from electrocatalytic water splitting.
Graphic Abstract
A novel cadmium nitrite initiated mesoporous carbon shell encapsulated binary cobalt/cobalt phosphide (Co/Co2P@MC-(Cdx)) composites were first prepared. The Co/Co2P@MC-(Cd0.5), with molar ratio of Cd/Co = 0.5 in the initial feeding stock, exhibited overpotentials of as low as 99 mV at 10 mA cm−2 and small Tafel slopes of 53 mV dec−1 under 0.5 M H2SO4 conditions. The superior activity of Co/Co2P@MC-(Cd0.5) was attributed to the introduction of Cd initiator, which resulted in the mesoporisity on carbon shell and the modified electron state between the Co and Co2P.










Similar content being viewed by others
References
Zou X, Zhang Y (2015) Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev 44:5148–5180
Popczun EJ, McKone JR, Read CG, Biacchi AJ, Wiltrout AM, Lewis NS, Schaak RE (2013) Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J Am Chem Soc 135:9267–9270
Xie J, Zhang H, Li S, Wang R, Sun X, Zhou M, Zhou J, Lou XW, Xie Y (2013) Defect-Rich MoS2 Ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv Mater 25:5807–5813
Wang L, Guo T, Sun S, Wang Y, Chen X, Zhang K, Zhang D, Xue Z, Zhou X (2019) Tree-Like NiS2/MoS2-RGO Nanocomposites as pH universal electrocatalysts for hydrogen evolution reaction. Catal Lett 149:1197–1210
Li Y, Huang H, Chen S, Wang C, Liu A, Ma T (2019) Killing two birds with one stone: a highly active tubular carbon catalyst with effective N doping for oxygen reduction and hydrogen evolution reactions. Catal Lett 149:486–495
Norskov JK, Bligaard T, Logadottir A, Kitchin JR, Chen JG, Pandelov S, Norskov JK (2005) Trends in the exchange current for hydrogen evolution. J Electrochem Soc 152:J23–J26
Zhang Y, Li C, Chen Z, Ni Y, Kong F, Kong A, Shan Y (2017) Ionic liquid-derived moc nanocomposites with ordered mesoporosity as efficient pt-free electrocatalyst for hydrogen evolution and oxygen reduction. Catal Lett 147:253–260
Wu J, He J, Li F, Hu X (2017) Ternary transitional metal chalcogenide nanosheet with significantly enhanced electrocatalytic hydrogen-evolution activity. Catal Lett 147:215–220
Mu J, Li J, Yang E-C, Zhao X-J (2018) Three-dimensional hierarchical nickel cobalt phosphide nanoflowers as an efficient electrocatalyst for the hydrogen evolution reaction under both acidic and alkaline conditions. Acs Appl Energy Mater 1:3742–3751
Fan X, Wang X, Yuan W, Li CM (2017) Diethylenetriamine-mediated self-assembly of three-dimensional hierarchical nanoporous CoP nanoflowers/pristine graphene interconnected networks as efficient electrocatalysts toward hydrogen evolution. Sustain Energy Fuels 1:2172–2180
Duan Z, Pi M, Zhang D, Zhang P, Deng J, Chen S (2019) High hydrogen evolution performance of Al doped CoP3 nanowires arrays with high stability in acid solution superior to Pt/C. Int J Hydrog Energy 44:8062–8069
Gao L, Chen S, Zhang H, Zou Y, She X, Yang D, Zhao Q, Zhao X (2018) Porous CoP nanostructure electrocatalyst derived from DUT-58 for hydrogen evolution reaction. Int J Hydrog Energy 43:13904–13910
Guo X, Liang J, Wang L, Feng Z, Yu T, Zhang Z, Shao Y, Hao C, Li G (2018) Synthesis of Cobalt-Glycerate hierarchical structure and their conversion into hierarchical CoP nanospheres for the hydrogen evolution reaction. Int J Hydrog Energy 43:2034–2042
Li Y, Li H, Cao K, Jin T, Wang X, Sun H, Ning J, Wang Y, Jiao L (2018) Electrospun three dimensional Co/CoP@nitrogen-doped carbon nanofibers network for efficient hydrogen evolution. Energy Storage Mater 12:44–53
Liu Q, Tian J, Cui W, Jiang P, Cheng N, Asiri AM, Sun X (2014) Carbon nanotubes decorated with CoP nanocrystals: a highly active non-noble-metal nanohybrid electrocatalyst for hydrogen evolution. Angew Chem Int Ed 53:6710–6714
Jiao L, Zhou Y-X, Jiang H-L (2016) Metal-organic framework-based CoP/reduced graphene oxide: high-performance bifunctional electrocatalyst for overall water splitting. Chem Sci 7:1690–1695
Lv Y, Gan L, Liu M, Xiong W, Xu Z, Zhu D, Wright DS (2012) A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J Power Sources 209:152–157
Hu ZH, Srinivasan MP, Ni YM (2001) Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 39:877–886
Larcher D, Sudant G, Patrice R, Tarascon JM (2003) Some insights on the use of polyols-based metal alkoxides powders as precursors for tailored metal-oxides particles. Chem Mater 15:3543–3551
Adam A, Suliman MH, Siddiqui MN, Yamani ZH, Merzougui B, Qamar M (2018) Interconnected hollow cobalt phosphide grown on carbon nanotubes for hydrogen evolution reaction. ACS Appl Mater Interfaces 10:29407–29416
Zhu Y, Fan X, Suo L, Luo C, Gao T, Wang C (2016) Electrospun FeS2@carbon fiber electrode as a high energy density cathode for rechargeable lithium batteries. ACS Nano 10:1529–1538
Guang L, Na L, Yong Z, Rui Y, Muheng W, Dongying H, Jinping L (2018) Fabrication of Fe-doped Co2P nanoparticles as efficient electrocatalyst for electrochemical and photoelectrochemical water oxidation. Electrochimica Acta 283:1490–1497
Mondal O, Mitra S, Datta A, Chakravorty D, Pal M (2016) Multifunctionality in graphene decorated with cobalt nanorods. Mater Des 101:204–209
Ryu J, Jung N, Jang JH, Kim H-J, Yoo SJ (2015) In situ transformation of hydrogen-evolving CoP nanoparticles: toward efficient oxygen evolution catalysts bearing dispersed morphologies with Co-oxo/hydroxo molecular units. ACS Catal 5:4066–4074
He L, Liu J, Liu Y, Cui B, Hu B, Wang M, Tian K, Song Y, Wu S, Zhang Z, Peng Z, Du M (2019) Titanium dioxide encapsulated carbon-nitride nanosheets derived from MXene and melamine-cyanuric acid composite as a multifunctional electrocatalyst for hydrogen and oxygen evolution reaction and oxygen reduction reaction. Appl Catal B 248:366–379
Wu S, Liu J, Cui B, Li Y, Liu Y, Hu B, He L, Wang M, Zhang Z, Tian K, Song Y (2019) Fluorine-doped nickel cobalt oxide spinel as efficiently bifunctional catalyst for overall water splitting. Electrochim Acta 299:231–244
Acknowledgments
This work was supported by the National Scientific Foundation of China (No: 21908242), the Natural Science Foundation of Jiangsu Province of China (No: BK20160239), the Foundation of Science and Technology Project of Xuzhou City (No: KC18063) and Natural Science Foundation of Guangdong Province (No. 2018A0303130212).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liang, H., Yang, C., Jia, L. et al. Mesoporous Carbon Shell Encapsulated Co/Co2P Composite for Electrocatalytic Hydrogen Evolution Reaction: The Effect of Cd Initiator on its Catalytic Performance. Catal Lett 150, 12–20 (2020). https://doi.org/10.1007/s10562-019-02930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02930-4