Abstract
Objective
To examine the association between maternal erythrocyte long-chain omega-3 PUFA (n-3 LCPUFA), measured in early pregnancy, and pregnancy and birth outcomes.
Study Design
One hundred and eight healthy women with a singleton pregnancy were included. Erythrocyte fatty acids were analyzed using gas chromatography. Gestational length, birth anthropometric measures, and pregnancy-associated complications were collected from hospital medical records.
Results
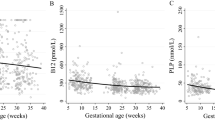
We observed significant positive associations between maternal docosahexaenoic acid (DHA) levels (p = 0.024) and omega-3 index values (p = 0.021) and gestational length in adjusted linear regression models. Each point in maternal DHA level was associated with 2.19 days longer gestational duration (β = 2.19; 95% CI 0.29–4.09). No consistent associations were found between n-3 PUFA levels and composite pregnancy outcome.
Conclusion
These findings suggest that the gestational length is positively affected by maternal n-3 LCPUFA status as soon as the early stages of pregnancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition.1997;13:807–13.
World Health Organization. Programming of chronic disease by impaired fetal nutrition. Geneva, Switzerland: World Health Organization; 2001.
WHO Regional Office for Europe. Good maternal nutrition: the best start in life. Copenhagen, Denmark: WHO Regional Office for Europe; 2016.
Innis SM. Metabolic programming of long-term outcomes due to fatty acid nutrition in early life. Matern Child Nutr. 2011;7:112–23.
Olsen SF, Hansen HS, Sørensen TI, Jensen B, Secher NJ, Sommer S, et al. Intake of marine fat, rich in (n-3)-polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet. 1986;2:367–9.
Leventakou V, Roumeliotaki T, Martinez D, Barros H, Brantsaeter A-L, Casas M, et al. Fish intake during pregnancy, fetal growth, and gestational length in 19 European birth cohort studies. Am J Clin Nutr. 2014;99:506–16.
Middleton P, Gomersall JC, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;11:CD003402.
Cinelli G, Fabrizi M, Ravà L, Signore F, Vernocchi P, Semeraro M, et al. Association between maternal and foetal erythrocyte fatty acid profiles and birth weight. Nutrients.2018;10:402.
Keelan JA, Newnham JP. Recent advances in the prevention of preterm birth. F1000Research. 2017;6:1139.
Newnham JP, Dickinson JE, Hart RJ, Pennell CE, Arrese CA, Keelan JA. Strategies to prevent preterm birth. Front Immunol. 2014;5:584.
Silva-Zolezzi I, Samuel TM, Spieldenner J. Maternal nutrition: opportunities in the prevention of gestational diabetes. Nutr Rev. 2017;75:32–50.
Burchakov D, Kuznetsova I, Uspenskaya Y. Omega-3 long-chain polyunsaturated fatty acids and preeclampsia: trials say “no,” but is it the final word? Nutrients. 2017;9:1364.
Lin P-Y, Chang C-H, Chong MF-F, Chen H, Su K-P. Polyunsaturated fatty acids in perinatal depression: a systematic review and meta-analysis. Biol Psychiatry. 2017;82:560–9.
Zhou SJ, Best K, Gibson R, McPhee A, Yelland L, Quinlivan J, et al. Study protocol for a randomised controlled trial evaluating the effect of prenatal omega-3 LCPUFA supplementation to reduce the incidence of preterm birth: the ORIP trial. BMJ Open. 2017;7:e018360.
Al MD, van Houwelingen AC, Kester AD, Hasaart TH, de Jong AE, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr. 1995;74:55–68.
Vlaardingerbroek H, Hornstra G. Essential fatty acids in erythrocyte phospholipids during pregnancy and at delivery in mothers and their neonates: comparison with plasma phospholipids. Prostaglandins Leukot Essent Fat Acids. 2004;71:363–74.
Dirix CEH, Kester AD, Hornstra G. Associations between neonatal birth dimensions and maternal essential and trans fatty acid contents during pregnancy and at delivery. Br J Nutr. 2008;101:399–407.
van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TG, Bonsel GJ. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr. 2008;87:887–95.
Hoge A, Tabar V, Donneau A-F, Dardenne N, Degée S, Timmermans M, et al. Imbalance between Omega-6 and Omega-3 polyunsaturated fatty acids in early pregnancy is predictive of postpartum depression in a Belgian cohort. Nutrients.2019;11:876.
Hoge A, Bernardy F, Donneau A-F, Dardenne N, Degée S, Timmermans M, et al. Low omega-3 index values and monounsaturated fatty acid levels in early pregnancy: an analysis of maternal erythrocytes fatty acids. Lipids Health Dis. 2018;17:63.
Harris WS, Von Schacky C. The Omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20.
Leroy C, Van Leeuw V, Zhang WH, Englert Y. Construction de l’indicateur « petit poids pour âge gestationnel » dans deux régions belges. In: Boithias C, Tolsa J-F, Chabernaud J-L, editors. Revue de médecine périnatale. Bruxelles, Belgium: Lavoisier; 2018;10:59–72.
IOM (Institute of Medicine), NRC (National Research Council). Weight gain during pregnancy: reexamining the Guidelines. Washington, D.C: National Academies Press; 2009.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–9.
Leroy C, Daelemans C, Debauche C, Van Leeuw V. Santé périnatale en Wallonie—Année 2017. Bruxelles: Centre d’Épidémiologie Périnatale asbl CEpiP; 2019.
Olsen SF, Secher NJ. Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study. BMJ.2002;324:447–50.
Olsen SF, Halldorsson TI, Thorne-Lyman AL, Strøm M, Gørtz S, Granstrøm C, et al. Plasma concentrations of long chain N-3 fatty acids in early and mid-pregnancy and risk of early preterm birth. EBioMedicine.2018;35:325–33.
Shireman TI, Kerling EH, Gajewski BJ, Colombo J, Carlson SE. Docosahexaenoic acid supplementation (DHA) and the return on investment for pregnancy outcomes. Prostaglandins Leukot Essent Fat Acids. 2016;111:8–10.
Jackson KH, Harris WS. A prenatal DHA test to help identify women at increased risk for early preterm birth: a proposal. Nutrients. 2018;10:1933.
Grootendorst-van Mil NH, Tiemeier H, Steenweg-de Graaff J, Koletzko B, Demmelmair H, Jaddoe VWV, et al. Maternal plasma n-3 and n-6 polyunsaturated fatty acids during pregnancy and features of fetal health: fetal growth velocity, birth weight and duration of pregnancy. Clin Nutr. 2018;37:1367–74.
Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-Hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010;140:999–1006.
Szajewska H, Horvath A, Koletzko B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2006;83:1337–44.
Chen B, Ji X, Zhang L, Hou Z, Li C, Tong Y. Fish oil supplementation improves pregnancy outcomes and size of the newborn: a meta-analysis of 21 randomized controlled trials. J Matern Neonatal Med. 2016;29:2017–27.
Carlson SE, Colombo J, Gajewski BJ, Gustafson KM, Mundy D, Yeast J, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808–15.
EFSA Panel on Dietetic Products Nutrition and Allergies. Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012;10:1–48.
Ordovas JM, Ferguson LR, Tai ES, Mathers JC. Personalised nutrition and health. BMJ. 2018;361:k2173.
Ogundipe E, Johnson MR, Wang Y, Crawford MA. Peri-conception maternal lipid profiles predict pregnancy outcomes. Prostaglandins Leukot Essent Fat Acids. 2016;114:35–43.
Markhus MW, Skotheim S, Graff IE, Frøyland L, Braarud HC, Stormark KM, et al. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS ONE. 2013;8:e67617.
Mohanty AF, Siscovick DS, Williams MA, Thompson ML, Burbacher TM, Enquobahrie DA. Periconceptional seafood intake and pregnancy complications. Public Health Nutr. 2016;19:1795–803.
Xie L, Innis SM. Genetic variants of the FADS1 FADS2 gene cluster are associated with altered (n-6) and (n-3) essential fatty acids in plasma and erythrocyte phospholipids in women during pregnancy and in breast milk during lactation. J Nutr. 2008;138:2222–8.
Meldrum SJ, Li Y, Zhang G, Heaton AEM, D’Vaz N, Manz J, et al. Can polymorphisms in the fatty acid desaturase (FADS) gene cluster alter the effects of fish oil supplementation on plasma and erythrocyte fatty acid profiles? An exploratory study. Eur J Nutr. 2018;57:2583–94.
Bernard JY, Pan H, Aris IM, Moreno-Betancur M, Soh S-E, Yap F, et al. Long-chain polyunsaturated fatty acids, gestation duration, and birth size: a mendelian randomization study using fatty acid desaturase variants. Am J Clin Nutr. 2018;108:92–100.
McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, et al. Evidence implicating peroxisome proliferator-activated receptor- in the pathogenesis of preeclampsia. Hypertension. 2011;58:882–7.
Wadhwani N, Patil V, Joshi S. Maternal long chain polyunsaturated fatty acid status and pregnancy complications. Prostaglandins Leukot Essent Fat Acids. 2018;136:143–52.
Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes. JAMA .2017;317:2207.
Hoge A, Bernardy F, Donneau A-F, Dardenne N, Degée S, Nisolle M, et al. Importance of n-3 PUFA consumption during pregnancy: perception discrepancies between pregnant women and gynaecologists-obstetricians in Belgium. Public Health Nutr. 2019;22:1259–68.
Acknowledgements
We would like to thank Florence Bernardy and Elisabetha Prato from the CHR Liège Hospital for their assistance in the data collection.
Funding
This research was funded by the medical analysis laboratory Roman Païs (Belgium) (http://www.rplab.be), Metagenics Europe (https://www.metagenics.eu) and University of Liège (Belgium) (https://www.uliege.be). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AH was involved in the research design and the coordination of the survey, and drafted the manuscript. She also conducted the data collection with the help of MT and under the supervision of MN and SD. MN and SD were also involved in the research design. AFD and ND performed the statistical analyses and took part in the critical discussion of the results. MN contributed to the critical revision and intellectual content of the manuscript. MG and VC as the promoters of the study supervised the entire work and finalized the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee of the CHR Citadelle Hospital of Liège, Belgium (B412201526650). Written informed consent was obtained from all pregnant women.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hoge, A., Donneau, AF., Dardenne, N. et al. Impact of erythrocyte long-chain omega-3 polyunsaturated fatty acid levels in early pregnancy on birth outcomes: findings from a Belgian cohort study. J Perinatol 40, 488–496 (2020). https://doi.org/10.1038/s41372-019-0573-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0573-9
This article is cited by
-
Low Omega-3 intake is associated with high rates of depression and preterm birth on the country level
Scientific Reports (2020)