Abstract
Study design:
Interventional feasibility study.
Objectives:
To evaluate safety and effects of local heat preconditioning on skin physiology using water-filtered infrared-A radiation (wIRA) or warm water therapy (wWT) in individuals with spinal cord injury (SCI).
Setting:
Acute and rehabilitation center, specialized in SCI.
Methods:
A convenience sample of 15 individuals (3 women, 12 men) with complete paraplegia from thoracic levels ranging between T2 and T12 received local heat applications either with wIRA or wWT on the thigh (paralyzed area) and on the upper arm (non-paralyzed area). Local heat was applied during three 30-min cycles, each separated by 30 min rest; thus, the treatment lasted for 180 min. Temperature, blood perfusion, and skin redness were measured at baseline, before and after heat application and 24 h after the last application.
Results:
Heat applications with wIRA and wWT were well-tolerated. No burns or any other side effects were detected. Skin temperature (p ≤ 0.008) and blood perfusion (p ≤ 0.013) significantly increased after heat application. Local skin temperature (arm p = 0.004/leg p < 0.001) and blood perfusion (arm p = 0.011/leg p = 0.001) after the first and the second application cycle, respectively, were significantly higher during heat application with wIRA than with wWT. However, skin redness did not change significantly (p = 0.1). No significant differences were observed between the paralyzed and non-paralyzed areas for all parameters immediately, as well as 24 h after the treatment.
Conclusions:
Although both heating methods have been confirmed as safe treatments in this study, further investigations with regard to their efficacy in the context of preconditioning are warranted.
Sponsorship:
The use of the instruments Hydrosun® 750 Irradiator (Hydrosun Medizintechnik, Germany) and Hilotherm-Calido 6 (Hilotherm GmbH, Germany) was sponsored by the Dr. med. h. c. Erwin Braun Foundation and by Hilotherm GmbH, respectively.
Similar content being viewed by others
Introduction
Individuals with spinal cord injury (SCI) display autonomic dysfunction due to a damage within the spinal sympathetic nervous pathways, this autonomic dysfunction is characterized by the absence of or the occurrence of abnormal sympathetic skin response [1, 2] and by the changes in biophysical skin properties, including skin perfusion and temperature control [3].
Reduction in skin perfusion and lack of vasodilatory responses after SCI is caused by a loss of sympathetic innervation [4, 5], leading to a substantial decrease in one’s capacity to respond to harmful stimuli and stress factors, such as heat exposures. Impaired tissue perfusion combined with a decrease in mobility and sensibility not only play an essential role in the etiology of pressure injury (PI) [6] but also affect wound healing. A high overall complication rate was reported during PI reconstruction with flap procedures in patients with SCI [7, 8]. Advanced stages of PIs in patients with SCI therefore frequently lead to a long hospitalization with surgical interventions, resulting in considerable health care costs and often reduced quality of life [9].
Therefore, clinically easily applicable and cost-effective strategies to avoid the effects of local microvascular dysfunction and ischemia are needed in order to prevent postoperative complications. Harder et al. showed that local heat preconditioning through warm water therapy (wWT) (Hilotherm-Calido 6), a simple and non-invasive method of tissue preconditioning, improved the skin-flap survival in a pig model [10, 11]. They reported an increased induction of heat shock proteins and a decrease in apoptosis. Also, thromboembolisation and subsequent microvascular perfusion failure was highly reduced by heat preconditioning [12]. In another clinical study involving patients undergoing skin-sparing mastectomy and immediate breast reconstruction—also a procedure with a relatively high complication rate due to ischemia—a 24% decrease in mastectomy flap necrosis was observed when preoperative local heat preconditioning was performed [13]. In addition to wWT, a new technology for local heat application involving water-filtered infrared therapy showed positive effects on wound healing and immunological repair mechanism, and this new technology does not involve skin contact [14]. However, the application of this technology in the context of flap surgery has never been examined.
Both methods have been used in a clinical setting in non-paralyzed patients, either for preconditioning before a flap procedure [13] or in wound healing after a laparotomy [15]. However, the effects of heat preconditioning on the skin of individuals with paraplegia remain unknown, and adverse effects due to the absence of sensitivity and due to autonomic dysfunction may occur.
Therefore, this feasibility study aims to evaluate the safety of local heat preconditioning in individuals with SCI, to investigate the changes in skin physiology in the paralyzed and non-paralyzed areas of the body, and to assess its effects on well-being, modulation of pain or spasticity. To this end, we used two different heating methods in otherwise healthy individuals with SCI.
Methods
Participants
This study included individuals presenting a complete sensory and motor SCI (with a motor score of 50) as measured by the international standard for neurological classification for SCI and with a lesion level ranging from thoracic T2 to T12. These patients had no control or sensation for bladder and bowel functioning. They were recruited at the post-acute phase (i.e., at least 12 weeks after SCI) or chronic phase (i.e., at least 12 months after SCI) of paralysis. Exclusion criteria included age <18 and >65 years, body temperature (i.e., forehead temperature) >38.5 °C, presence of an acute infection, diabetes mellitus, stage III heart failure, stage III renal insufficiency, presence of wounds or scars in the skin area to be heated, and skin type V (light brown) or VI (brown) as defined by Fitzpatrick [16].
Setting
The patients were recruited during a hospital stay at an acute and rehabilitation center specialized in SCI. Due to organizational reasons, feasibility investigations had to be operated in two phases. The first and second heating cycles involving wIRA (Hydrosun® 750 irradiator) and wWT (Hilotherm-Calido 6), respectively, were conducted in a standardized examination room acclimatized to 24 °C.
Heat sources
Two different devices were used for the local heat application. The first study group (wIRA group) was treated with wIRA by using a Hydrosun® 750 Irradiator (Hydrosun Medizintechnik, Germany). This device emits water-filtered infrared-A radiation (spectral range: 760–1400 nm). It is a non-contact heating method adjusted at 43 °C with a distance set at 50 cm from the skin surface.
The second study group (wWT group) was treated using a Hilotherm-Calido 6 (Hilotherm GmbH, Germany). Heating was applied via moldable silicone cuffs, which were applied directly on the skin. The temperature of the water flowing through these cuffs was kept at 43 °C.
Subjective evaluation
Patients were asked to complete a questionnaire after the intervention to evaluate their perception of change in general well-being, pain, and spasticity based on a visual analog scale (VAS) of 0–10. We defined 0 as “good” well-being or “absence” of pain or spasticity and 10 as “bad” well-being or “severe” pain and spasticity. There were no objective evaluation of pre-test spasticity in our study.
Objective evaluation
An infrared camera (Thermovision A40, Orgelmeister Infrarot-Systeme, Germany) was used for the non-contact skin temperature measurements. Measurements were obtained continuously in the wIRA group. In the wWT group, a continuous measurement of skin temperature was impossible as the local skin was covered with silicone cuffs during heat application. Skin perfusion was assessed using laser Doppler technology (PeriFlux System 5000, Perimed, Sweden) within 90 s, and skin redness was assessed with a Mexameter MX18 (Courage-Khazaka Electronic, Germany).
We measured blood pressure (Riva–Roci method), pulse (manually), and body temperature (based on the forehead skin temperature; Scaneo Thermometer, Sanowell, Germany); these parameters are related to the responses of the autonomic nervous system.
Clinical evaluations of adverse effects, such as blister formation and any other signs of skin burns, were performed at the end of the last heating cycle and 24 h later. Skin color was documented through photographs. Redness was assessed as either without redness or with slight or intense redness.
Study protocol
After being acclimatized during 30 min at a constant room temperature of 24 °C, the patients underwent three 30-min cycles of local heat application simultaneously in their non-paralyzed upper extremity and in their paralyzed lower extremity. Each 30-min cycle of heat application was followed by a 30-min resting phase to allow spontaneous cooling of the treated areas (Fig. 1). No further methods to initiate local cooling were applied.
During the acclimatization phase, both body sites to be used for local heat application were marked. At baseline, after the third cycle (T5) and in the morning on the next day (D2), pulse, blood pressure, and body temperatures were measured. Additionally, skin redness and blood perfusion were measured in both heated areas at these times. Subjective parameters were assessed before the intervention, at the end of day 1 (T5) and on D2.
Statistics
The median and 95% confidence intervals were calculated based on the data obtained from 10 individuals assigned to each group. We performed an independent comparison of interventions in the patients whether or not they received both heating methods. The effect of group assignment (wWT/wIRA) (between-effect), location (shoulder/thigh) (within-effect), and time point (within-effect) on the skin parameters were investigated using non-parametric (rank-based) repeated-measures multivariate analysis of variance (Brunner Model) based on marginal distribution effects [17]. We calculated between-effects and within-effects, as well as interactions by means of the marginal distributions. The Wilcoxon ranked-sum test was used to investigate the differences between the time points for the two locations and groups (post-hoc testing). The Mann–Whitney U test was used to investigate the differences between the groups at the specific time points (post-hoc testing). Statistical analyses were performed using the R software environment (version 3.4.0, Copyright 2017, The R Foundation for Statistical Computing) and the package “nparLD”. Furthermore, the SPSS software (version 25, IBM, Somers, NY, USA) was used. A p-value of ≤0.05 was considered significant.
Results
Fifteen patients were included in this study. Ten participants received the wIRA during the first measurement phase. During the second phase, 10 participants received the wWT; thus 10 data sets were obtained from each group. Five participants received both heating methods. All included patients have completed the intervention. Intervention with wIRA took place between October 2016 and January 2017, whereas wWT was applied between February and April in 2017. Table 1 shows the patient characteristics.
Clinical examination for adverse effects
No adverse effects, such as blisters or other signs of burn injuries or skin irritation, occurred during the entire investigation in both locations and with the use of both devices.
Well-being, pain, and spasticity
One patient in the wWT (VAS of 5) complained about pain and increased spasticity during the experiment, and one patient in the wIRA group (VAS of 5) complained about decreased well-being. Nevertheless, the heat therapy was overall well-tolerated, and the heating did not lead to significant changes in well-being (Table 2).
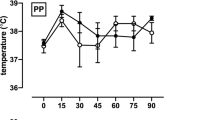
Local skin temperature
Local skin temperature values are presented in Fig. 2 and Table 3. The baseline temperature of the two groups did not differ significantly (p = 0.14). After local heating and after all heating cycles, skin temperature increased significantly (p < 0.001) compared with the baseline in both groups. Temperature at the upper extremity was significantly higher (p ≤ 0.0003) in the wIRA group than in the wWT group after each heating cycle. Temperature at the lower extremity was significantly (p < 0.0001) higher in the wIRA group than in the wWT group only after the second heating cycle. At the lower extremity site, the temperature significantly increased (p < 0.002) after the second heating cycle and stabilized at the same level during the third heating cycle. No significant difference (p = 0.80) in temperatures was observed between the treatment sites within one individual in the paralyzed lower extremity versus the non-paralyzed upper extremity. Between heating cycles, the temperature did not significantly differ (p > 0.05) from the baseline.
TWT thermal warm water therapy, wIRA water-filtered infrared-A radiation therapy, UE upper arm, LE thigh, *significantly different from the value obtained before the heating cycle (Wilcoxon ranked-sum test p ≤ 0.05); †significantly different from the wWT group (Mann–Whitney U test p ≤ 0.004) (with 95% confidence interval).
Perfusion
The heating method (p = 0.0008) and time point (p < 0.0001) had a significant effect on skin perfusion. The location had no significant (p = 0.83) effect. Local heating resulted in a significant increase (p < 0.001) in skin perfusion comparing to the baseline regardless of the heating method used (Table 3, Fig. 3). After completing of the first heating cycle, perfusion was significantly higher (p < 0.011) in the wIRA group than in the wWT group. After the second and third heating cycles, no significant differences in skin perfusion were observed between the two groups (p = 0.089) and between the upper and lower extremities sites (p = 0.50).
TWT thermal warm water therapy, wIRA water-filtered infrared-A radiation therapy, UE upper arm, LE thigh, *significantly different from the value obtained before the heating cycle (Wilcoxon ranked-sum test p ≤ 0.05); †significantly different from the TWT group (Mann–Whitney U test p ≤ 0.011) (with 95% confidence interval).
Redness
The heating method (p = 0.018) and location (p < 0.0001) had a significant effect on redness. However, no significant difference was localized between the upper and lower extremities (p > 0.05), between the two groups (p > 0.08) and the time points (p = 0.11) (Table 3, Fig. 4).
Body temperature, heart rate, and pulse
Heating did not result in any relevant changes in body temperature, heart rate, or pulse rate during the entire experiment (Table 3).
Discussion
Although wIRA is a safe treatment in wound healing [15, 18, 19], its effect on paralyzed tissue or skin flaps and on heat shock preconditioning remains unknown. Furthermore, the wWT methodology is relatively new with minimal scientific data available. Therefore, this study primarily aimed to investigate the feasibility of both methodologies for local heat application in individuals with complete paraplegia. In both treatment groups, no adverse effects, such as burns, blisters, or any other signs of skin injury, occurred. This finding was corroborated by the mexameter assessment of skin redness, demonstrating the absence of significant changes after local heat application. Subjective evaluation of the two heating methods revealed a good tolerance among the patients, with only a few patients reporting an increase in pain (one patient) and spasticity (one patient). Only one patient reported a decrease in well-being during the procedure. Because we conducted only a subjective evaluation of changes in spasticity directly after treatment, these results are indicative and have to be interpreted with caution.
In the baseline perfusion examination, the values obtained on the thigh [20.5 IU (11.9–33.5)] and shoulder [19.1 IU (18.1–37.0)] were higher than those obtained over the sacrum of healthy persons [17.1 IU (12.4; 21.5)] [20]. Moreover, higher values [27.7 IU (21.2; 40.2)] were obtained in healthy people with SCI when measurements were taken in the morning right after sleep [21]. In our groups, the redness values on the shoulder [265.7 IU (218.2–317.8)] and thigh [217.8 IU (133.2–304.6)] were also higher than the values taken over the sacrum in healthy persons [158.5 (126.3; 190.5)] and in individuals with SCI [206.5 IU (182.1; 250.1)] [21].
Redness 595.5 IU [440.4; 631.6] and perfusion 263.0 IU [104.1; 659.4] values over PI grade 1 in individuals with SCI were higher than the values obtained after performing both heating interventions [redness 278.8 IU (233.0–371.8) for shoulder and thigh 206.8 (138.0–323.4) and perfusion for shoulder 128.0 IU (101.9–174.7) and thigh 137.7 IU (84.1–250.9)]. The measurements obtained using the different examination techniques greatly varied due to individual skin reaction or due to measurement uncertainty [20].
Expectedly, the temperature increased significantly after local heating of the skin in both the wIRA and wWT groups. With wIRA, significantly higher temperatures were obtained mainly in the upper extremity than in the lower extremity. Body temperature did not change when the two zones were heated. One possible reason for this slight difference in temperature rise between the wIRA and wWT treatments is the fact that two direct heating sources were used in the wIRA group, whereas one device with two long connecting tubing was used in the wWT group. The surface of the cuffs and the length of the connecting tubing might have caused some energy loss; also, the device may not have heated the skin homogenously. Furthermore, the application of the wIRA as a non-contact intervention revealed some practical advantages with regard to the ease of heat application and patient-burden without forfeits in heating effects.
The effect of the impaired autonomic nervous system on changes in perfusion after local heating is attributed to various reasons. Nicotra observed a reduced reaction to heating in the paraplegic foot area compared with controls after heating [22]. In our study, we observed an increase in local skin perfusion by the same extent in the sensitive and paralyzed areas. The variability in participants with higher SCI lesion levels is in line with previous findings about differences in sympathetic skin response [23]. However, heating mediated vasodilation and increased blood flow in both areas, as measured by the laser Doppler flowmeter Perfusion is regulated by the sympathetic nervous system, as well as by the monosynaptic and complex polysynaptic reflexes [24]. The autonomic dysfunction seemed to have less effect on skin perfusion than the local heat application itself, which may have a direct physical effect on the vascular wall and local physiological changes. Our results indicate probably a reduced resistance to external stimuli as demonstrated by Krassioukov [25]. Alternatively, these results indicate that the local heat stimuli overlap with the influence of the autonomic nervous system and might affect the efficacy of the heating methodology applied. As for temperature, the effect of wIRA on perfusion was also slightly greater than that of wWT, although both methods significantly increased blood perfusion.
Although skin physiology in the paralyzed area is supposedly different in terms of biomechanical properties [26, 27], autonomic functions [28] and thermoregulation [29, 30], no significant temperature differences between the affected and the unaffected areas were detected at any time of the intervention. Since both methods were well-tolerated, it is possible that local heating did not trigger any physiologic response controlling local temperature; and thus, no differences between the paralyzed and the non-paralyzed areas could be observed. Furthermore, it is possible that local blood perfusion was maximally increased by the heating or that direct vascular reactions to the stimulation are not mediated by any mechanisms affected by the SCI.
In previous pre-clinical and clinical models of heat preconditioning, local heat was applied ~24 h before an ischemic challenge, such as a flap surgery [10, 11, 31]. Initially, this interval was chosen to allow sufficient time for the upregulation of physiological pathways to facilitate response to external heat applications. However, whether the beneficial effect of local heat shock preconditioning is linked to the prevention of microcirculatory failure or to the induction of tolerance to ischemia remains unknown [31]. In these previous experiments, perfusion before the induction of ischemia did not increase, and perfusion values also returned to the baseline after the preconditioning and before the ischemia/flap surgery. Whether heat shock proteins are upregulated or whether another repair mechanism was activated to reduce the occurrence of wound healing complications after flap surgery remains unclear and should be examined in further clinical studies.
Limitations
This study is the first to evaluate the safety of and the physiological responses to two established heat shock preconditioning methods which have never been used in individuals with SCI. As a pilot study and given the small number of participants, caution must be exercised when interpreting the conclusion and when making generalizations. Especially, the subjective evaluation of the methods might be biased due to the lack of blinding to the application methods. Furthermore, subjective feedback was obtained for perceived changes in well-being, pain, and spasticity after the intervention rather than asking for the actual condition before and after the test. Because our primary outcome was safety and physiological response of the skin, the bias was judged as not relevant. Due to the moderate reliability of the measurement methods for perfusion and redness, the quality of the measured data remains limited.
Five individuals received both heat treatments and ten individuals received either wWT or wIRA treatment (n = 5). Thus, 10 data sets for each heat treatment were obtained. It was impossible to fully respect the within-effects and between-effects in the multifactorial repeated measures analysis of variance used. The factor heat treatment was analyzed as between-effect, even though this was not the case in half of the data sets. This approach increased the type II error probability. However, this work is a feasibility study, which did not aim to achieve maximum statistical power.
Conclusion
The result of this pilot study on the safety of heat application in a paralyzed area after SCI demonstrated that heat application was well-tolerated in the participants with complete paraplegia and that the heat application did not cause any adverse effects or complications, such as burn injuries. Local temperature and perfusion significantly increased after heating with wIRA or with wWT. Whether heat shock preconditioning can be used to decrease the complication rate of reconstructive surgical procedures in individuals with SCI and which methodology reaches higher treatment efficacy remains to be determined.
Data archiving
The data analyzed during this study are available from the corresponding author on reasonable request.
References
Berger MJ, Hubli M, Krassioukov AV. Sympathetic skin responses and autonomic dysfunction in spinal cord injury. J Neurotrauma. 2014;31:1531–9.
Jan Y-K, et al. Comparison of changes in heart rate variability and sacral skin perfusion in response to postural changes in people with spinal cord injury. J Rehabilitation Res Dev. 2013;50:203.
Scheel-Sailer A, et al. Biophysical skin properties of grade 1 pressure ulcers and unaffected skin in spinal cord injured and able-bodied persons in the unloaded sacral region. J Tissue Viability. 2017;26:89–94.
Fuyuan L, Yih-Kuen J. Using multifractal detrended fluctuation analysis to assess sacral skin blood flow oscillations in people with spinal cord injury. J Rehabilitation Res Dev. 2011;48:787–99.
Liao F, Burns S, Jan YK. Skin blood flow dynamics and its role in pressure ulcers. J Tissue Viability. 2013;22:25–36.
Makhsous M, et al. Measuring tissue perfusion during pressure relief maneuvers: insights into preventing pressure ulcers. J Spinal Cord Med. 2007;30:497–507.
Wettstein R, et al. Local flap therapy for the treatment of pressure sore wounds. Int Wound J. 2015;12:572–6.
Kreutztrager M, et al. Outcome analyses of a multimodal treatment approach for deep pressure ulcers in spinal cord injuries: a retrospective cohort study. Spinal Cord. 2018;56:582–90.
DeVivo M, Farris V. Causes and costs of unplanned hospitalizations among persons with spinal cord injury. topics in spinal cord injury. Rehabilitation. 2011;16:53–61.
Harder Y, et al. Heat shock preconditioning reduces ischemic tissue necrosis by heat shock protein (HSP)-32-mediated improvement of the microcirculation rather than induction of ischemic tolerance. Ann Surg. 2005;242:869.
Contaldo C, et al. The influence of local and systemic preconditioning on oxygenation, metabolism and survival in critically ischaemic skin flaps in pigs. J Plast Reconstructive Aesthetic Surg. 2007;60:1182–92.
Rücker M, et al. Local heat shock priming promotes recanalization of thromboembolized microvasculature by upregulation of plasminogen activators. Arteriosclerosis Thrombosis Vasc Biol. 2006;26:1632–9.
Mehta S, et al. Local heat preconditioning in skin sparing mastectomy: a pilot study. J Plast Reconstructive Aesthetic Surg. 2013;66:1676–82.
Hoffmann G, Hartel M, Mercer JB. Heat for wounds–water-filtered infrared-A (wIRA) for wound healing–a review. GMS German Med Sci. 2016;14:1–22.
Künzli BM, et al. Impact of preoperative local water-filtered infrared A irradiation on postoperative wound healing: a randomized patient-and observer-blinded controlled clinical trial. Ann Surg. 2013;258:887–94.
Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. JAMA Dermatol. 1988;124:869–71.
Brunner E, Langer F. Nonparametric analysis of ordered categorical data in designs with longitudinal observations and small sample sizes. Biometrical J. 2000;42:663–75.
Winkel R, Hoffmann G, Hoffmann R. [Water-filtered infrared-A (wIRA) promotes wound healing]. der Chir Z fur alle Geb der operativen Medizen. 2014;85:980–92.
Hartel M, et al. Randomized clinical trial of the influence of local water‐filtered infrared A irradiation on wound healing after abdominal surgery. Br J Surg. 2006;93:952–60.
Scheel-Sailer A, et al. Challenges to measure hydration, redness, elasticity and perfusion in the unloaded sacral region of healthy persons after supine position. J Tissue Viability. 2015;24:62–70.
Scheel-Sailer A, et al. Biophysical skin properties of grade 1 pressure ulcers and unaffected skin in spinal cord injured and able-bodied persons in the unloaded sacral region. J Tissue Viability. 2017;26:89–94.
Nicotra A, Asahina M, Mathias C. Skin vasodilator response to local heating in human chronic spinal cord injury. Eur J Neurol. 2004;11:835–7.
Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma. 2006;23:1713–25.
Lehmann J, de Lateur B. Application of heat and cold in the clinical setting. In: Lehmann J. F. editor. Therapeutic Heat and Cold. 4th edn. Baltimore: Williams & Wilkins; 1990. pp. 633–44.
Coombs GB, et al. Acute heat stress reduces biomarkers of endothelial activation but not macro- or microvascular dysfunction in cervical spinal cord injury. Am J Physiol Heart Circulatory Physiol. 2019;316:H722–33.
Mak AF, Zhang M, Tam EW. Biomechanics of pressure ulcer in body tissues interacting with external forces during locomotion. Annu Rev Biomed Eng. 2010;12:29–53.
Choi JH, et al. Generation of viable embryos and embryonic stem cell-like cells from cultured primary follicles in mice1. Biol Reprod. 2011;85:744–54.
Previnaire JG, et al. Severity of autonomic dysfunction in patients with complete spinal cord injury. Clin Autonomic Res. 2012;22:9–15.
Krassioukov A. Autonomic function following cervical spinal cord injury. Respiratory Physiol Neurobiol. 2009;169:157–64.
Hou S. Autonomic consequences of spinal cord injury. Compr Physiol. 2014;4:1419–53.
Harder Y, et al. Improved skin flap survival after local heat preconditioning in pigs1. J Surgical Res. 2004;119:100–5.
Acknowledgements
We would like to thank all the participants and the patients at the Swiss Paraplegic Centre.
Funding
We would like to thank the Dr. h.c. Erwin Braun Stiftung and the HiloTherm GmbH for providing the instruments used in this study.
Author information
Authors and Affiliations
Contributions
AS-S: study design, data analysis, experimentation, paper preparation, study monitoring. NA: study design, establishment of methods, experimentation, data analysis, paper preparation. DJ: experimentation, data analysis, paper preparation. This study serves as his Master’s Thesis in Medicine at the University of Basel. AW: study design, establishment of methods, experimentation. This study serves as her Master’s Thesis in Medicine at the University of Basel. SA: study design, establishment of methods, data analysis, paper preparation. YH: study design, establishment of methods, manuscript preparation. JK: study design, data analysis and statistics, paper preparation. RW: study design, data analysis, study monitoring, paper preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of ethics
The study protocol was approved by the Ethics Committee of the Northwest and Central Switzerland (ECNZ 2016–01158) and was registered on clinicaltrial.gov (2014–05 NCT03001531). Each patient signed the consent declaration before undergoing the intervention. This study was conducted following the research principles of good clinical practice.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Scheel-Sailer, A., Aliyev, N., Jud, D. et al. Changes in skin-physiology after local heat application using two different methods in individuals with complete paraplegia: a feasibility and safety trial. Spinal Cord 58, 667–674 (2020). https://doi.org/10.1038/s41393-019-0408-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0408-8
This article is cited by
-
Central versus peripheral mechanisms of cold-induced vasodilation: a study in the fingers and toes of people with paraplegia
European Journal of Applied Physiology (2023)