Abstract
Purpose
Basal/acetazolamide brain perfusion single-photon emission computed tomography (SPECT) has been used to evaluate functional hemodynamics in patients with carotid artery stenosis. We aimed to develop a deep learning model as a support system for interpreting brain perfusion SPECT leveraging unstructured text reports.
Methods
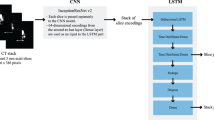
In total, 7345 basal/acetazolamide brain perfusion SPECT images and their text reports were retrospectively collected. A long short-term memory (LSTM) network was trained using 500 randomly selected text reports to predict manually labeled structured information, including abnormalities of basal perfusion and vascular reserve for each vascular territory. Using this trained LSTM model, we extracted structured information from the remaining 6845 text reports to develop a deep learning model for interpreting SPECT images. The model was based on a 3D convolutional neural network (CNN), and the performance was tested on the other 500 cases by measuring the area under the receiver-operating characteristic curve (AUC). We then applied the model to patients who underwent revascularization (n = 33) to compare the estimated output of the CNN model for pre- and post-revascularization SPECT and clinical outcomes.
Results
The AUC of the LSTM model for extracting structured labels was 1.00 for basal perfusion and 0.99 for vascular reserve for all 9 brain regions. The AUC of the CNN model designed to identify abnormal perfusion was 0.83 for basal perfusion and 0.89 for vascular reserve. The output of the CNN model was significantly improved according to the revascularization in the target vascular territory, and its changes in brain territories were concordant with clinical outcomes.
Conclusion
We developed a deep learning model to support the interpretation of brain perfusion SPECT by converting unstructured text reports into structured labels. This model can be used as a support system not only to identify perfusion abnormalities but also to provide quantitative scores of abnormalities, particularly for patients who require revascularization.





Similar content being viewed by others
References
Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: can acetazolamide test predict it? Stroke. 2001;32:2110–6 https://doi.org/10.1161/hs0901.095692.
Noh HJ, Kim SJ, Kim JS, Hong SC, Kim KH, Jun P, et al. Long term outcome and predictors of ischemic stroke recurrence in adult moyamoya disease. J Neurol Sci. 2015;359:381–8 https://doi.org/10.1016/j.jns.2015.11.018.
Knop J, Thie A, Fuchs C, Siepmann G, Zeumer H. 99mTc-HMPAO-SPECT with acetazolamide challenge to detect hemodynamic compromise in occlusive cerebrovascular disease. Stroke. 1992;23:1733–42 https://doi.org/10.1161/01.str.23.12.1733.
Tomura N, Otani T, Koga M, Ishiyama K. Correlation between severity of carotid stenosis and vascular reserve measured by acetazolamide brain perfusion single photon emission computed tomography. J Stroke Cerebrovasc Dis. 2013;22:166–70. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.07.011.
Touho H, Karasawa J, Ohnishi H. Preoperative and postoperative evaluation of cerebral perfusion and vasodilatory capacity with 99mTc-HMPAO SPECT and acetazolamide in childhood Moyamoya disease. Stroke. 1996;27:282–9. https://doi.org/10.1161/01.str.27.2.282.
Ilyas A, Chen CJ, Ironside N, Buell TJ, Chagoya G, Schmalz PG, et al. Medical management versus surgical bypass for symptomatic intracranial atherosclerotic disease: a systematic review. World Neurosurg. 2019. https://doi.org/10.1016/j.wneu.2019.05.223.
Van Laere KJ, Warwick J, Versijpt J, Goethals I, Audenaert K, Van Heerden B, et al. Analysis of clinical brain SPECT data based on anatomic standardization and reference to normal data: an ROC-based comparison of visual, semiquantitative, and voxel-based methods. J Nucl Med. 2002;43:458–69.
Schmittling ZC, McLafferty RB, Danetz JS, Hussain SM, Ramsey DE, Hodgson KJ. The inaccuracy of simple visual interpretation for measurement of carotid stenosis by arteriography. J Vasc Surg. 2005;42:62–6. https://doi.org/10.1016/j.jvs.2005.03.028.
Saito N, Nakagawara J, Nakamura H, Teramoto A. Assessment of cerebral hemodynamics in childhood moyamoya disease using a quantitative and a semiquantitative IMP-SPECT study. Ann Nucl Med. 2004;18:323–31. https://doi.org/10.1007/bf02984471.
Choi H, Yoo MY, Cheon GJ, Kang KW, Chung JK, Lee DS. Parametric cerebrovascular reserve images using acetazolamide 99mTc-HMPAO SPECT: a feasibility study of quantitative assessment. Nucl Med Mol Imaging. 2013;47:188–95. https://doi.org/10.1007/s13139-013-0214-8.
Lee HY, Paeng JC, Lee DS, Lee JS, Oh CW, Cho MJ, et al. Efficacy assessment of cerebral arterial bypass surgery using statistical parametric mapping and probabilistic brain atlas on basal/acetazolamide brain perfusion SPECT. J Nucl Med. 2004;45:202–6.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44. https://doi.org/10.1038/nature14539.
Shen D, Wu G, Suk HI. Deep learning in medical image analysis. Annu Rev Biomed Eng. 2017;19:221–48. https://doi.org/10.1146/annurev-bioeng-071516-044442.
Choi H. Deep learning in nuclear medicine and molecular imaging: current perspectives and future directions. Nucl Med Mol Imaging. 2018;52:109–18. https://doi.org/10.1007/s13139-017-0504-7.
Gao S, Young MT, Qiu JX, Yoon HJ, Christian JB, Fearn PA, et al. Hierarchical attention networks for information extraction from cancer pathology reports. J Am Med Inform Assoc. 2017. https://doi.org/10.1093/jamia/ocx131.
Banerjee I, Gensheimer MF, Wood DJ, Henry S, Aggarwal S, Chang DT, et al. Probabilistic prognostic estimates of survival in metastatic cancer patients (PPES-met) utilizing free-text clinical narratives. Sci Rep. 2018;8:10037. https://doi.org/10.1038/s41598-018-27946-5.
Gomez L, Patel Y, Rusiñol M, Karatzas D, Jawahar CV. Self-supervised learning of visual features through embedding images into text topic spaces. In: IEEE conference on computer vision and pattern recognition; 2017. p. 2017–26.
Triguero I, García S, Herrera FJK, Systems I. Self-labeled techniques for semi-supervised learning: taxonomy, software and empirical study. Knowl Inf Syst. 2015;42:245–84. https://doi.org/10.1007/s10115-013-0706-y.
Jacobs F, Thierens H, Piepsz A, Bacher K, Van de Wiele C, Ham H, et al. Optimised tracer-dependent dosage cards to obtain weight-independent effective doses. Eur J Nucl Med Mol Imaging. 2005;32:581–8. https://doi.org/10.1007/s00259-004-1708-5.
Kingma DP. Ba J. A method for stochastic optimization. arXiv: Adam; 2014. https://arxiv.org/pdf/1412.6980.pdf
Reynolds MR, Derdeyn CP, Grubb RL Jr, Powers WJ, Zipfel GJ. Extracranial-intracranial bypass for ischemic cerebrovascular disease: what have we learned from the carotid occlusion surgery study? Neurosurg Focus. 2014;36:E9. https://doi.org/10.3171/2013.10.Focus13427.
White TG, Abou-Al-Shaar H, Park J, Katz J, Langer DJ, Dehdashti AR. Cerebral revascularization after the carotid occlusion surgery study: what candidates remain, and can we do better? Neurosurg Focus. 2019;46:E3. https://doi.org/10.3171/2018.11.Focus18536.
Song YS, Oh SW, Kim YK, Kim SK, Wang KC, Lee DS. Hemodynamic improvement of anterior cerebral artery territory perfusion induced by bifrontal encephalo(periosteal) synangiosis in pediatric patients with moyamoya disease: a study with brain perfusion SPECT. Ann Nucl Med. 2012;26:47–57. https://doi.org/10.1007/s12149-011-0541-8.
Kim SK, Seol HJ, Cho BK, Hwang YS, Lee DS, Wang KC. Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery. 2004;54:840–4. https://doi.org/10.1227/01.neu.0000114140.41509.14.
Kim SK, Wang KC, Kim IO, Lee DS, Cho BK. Combined encephaloduroarteriosynangiosis and bifrontal encephalogaleo(periosteal)synangiosis in pediatric moyamoya disease. Neurosurgery. 2002;50:88–96. https://doi.org/10.1097/00006123-200201000-00016.
Ito H, Inoue K, Goto R, Kinomura S, Taki Y, Okada K, et al. Database of normal human cerebral blood flow measured by SPECT: I. comparison between I-123-IMP, Tc-99m-HMPAO, and Tc-99m-ECD as referred with O-15 labeled water PET and voxel-based morphometry. Ann Nucl Med. 2006;20:131–8. https://doi.org/10.1007/bf02985625.
Lee JS, Lee DS, Kim YK, Kim J, Lee HY, Lee SK, et al. Probabilistic map of blood flow distribution in the brain from the internal carotid artery. Neuroimage. 2004;23:1422–31. https://doi.org/10.1016/j.neuroimage.2004.07.057.
Kim SJ, Kim IJ, Kim YK, Lee TH, Lee JS, Jun S, et al. Probabilistic anatomic mapping of cerebral blood flow distribution of the middle cerebral artery. J Nucl Med. 2008;49:39–43. https://doi.org/10.2967/jnumed.107.045724.
Jagannatha AN, Yu H. Structured prediction models for RNN based sequence labeling in clinical text. In: conference on empirical methods in natural language processing; 2016. p. 856–65.
Funding
This study was supported by the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Korean government, MSIP (2017M3C7A1048079) and supported by the NRF funded by the Korea government (MSIT) (NRF-2019R1F1A1061412).
Author information
Authors and Affiliations
Contributions
Conceptualization: Hyun Gee Ryoo, Hongyoon Choi, Dong Soo Lee; Methodology: Hyun Gee Ryoo, Hongyoon Choi; Software: Hyun Gee Ryoo, Hongyoon Choi; Formal analysis and investigation: Hyun Gee Ryoo; Writing original draft preparation: Hyun Gee Ryoo; Writing, review, and editing: Hongyoon Choi, Dong Soo Lee; Supervision: Dong Soo Lee.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective study was approved by Seoul National University/Seoul National University Hospital Institutional Review Board. The needs for informed consent were waived by the committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence).
Electronic supplementary material
ESM 1
(PDF 966 kb)
Rights and permissions
About this article
Cite this article
Ryoo, H.G., Choi, H. & Lee, D.S. Deep learning-based interpretation of basal/acetazolamide brain perfusion SPECT leveraging unstructured reading reports. Eur J Nucl Med Mol Imaging 47, 2186–2196 (2020). https://doi.org/10.1007/s00259-019-04670-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04670-4