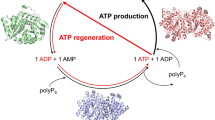
Abstract
Enzymes used for adenosine triphosphate (ATP) synthesis play important roles in energy-dependent cascade reactions in vitro. In this study, two novel polyphosphate kinase 2 (PPK2) enzymes, HbPPK2 from Hydrogenophilaceae bacterium and NdPPK2 from Nocardioides dokdonensis, were characterized for ATP synthesis with the substrate polyphosphate (polyP). The optimum temperature and pH of both purified HbPPK2 and NdPPK2 were 30 °C and 6.5. HbPPK2 and NdPPK2 retained 30% and 14% of the initial activity at 30 °C for 12 h, respectively, whereas the presence of polyP significantly enhanced the stability of enzymes. The two PPK2s preferentially catalyzed the long-chain polyP hexametaphosphate as the phosphate donor. Adenosine monophosphate could not be used by HbPPK2 and NdPPK2 to synthesize ATP, indicating that they belonged to the class I subfamily of PPK2. HbPPK2 was used for ATP regeneration to produce glutathione by a two-enzyme cascade in vitro. 47.1 ± 0.4 mM glutathione was synthesized with a productivity of 13.5 ± 0.1 mM/h. ATP was regenerated approximately 471 times in the system within 3.5 h. HbPPK2 showed potential application for ATP regeneration in cascade reaction.






Similar content being viewed by others
References
Schrittwieser, J. H., Velikogne, S., Hall, M., & Kroutil, W. (2017). Artificial biocatalytic linear cascades for preparation of organic molecules. Chemical Reviews, 118(1), 270–348.
Chen, Z., & Zeng, A. P. (2016). Protein engineering approaches to chemical biotechnology. Current Opinion in Biotechnology, 42, 198–205.
Wohlgemuth, R., Liese, A., & Streit, W. (2017). Biocatalytic phosphorylations of metabolites: Past, present, and future. Trends in Biotechnology, 35(5), 452–465.
Andexer, J. N., & Richter, M. (2015). Emerging enzymes for ATP regeneration in biocatalytic processes. ChemBioChem, 16(3), 380–386.
Zhao, H., & Donk, W. A. V. D. (2003). Regeneration of cofactors for use in biocatalysis. Current Opinion in Biotechnology, 14, 583–589.
Sperl, J. M., & Sieber, V. (2018). Multienzyme cascade reactions-status and recent advances. ACS Catalysis, 8(3), 2385–2396.
Kornberg, A. (1995). Inorganic polyphosphate: Toward making a forgotten polymer unforgettable. Journal of Bacteriology, 177(3), 491–496.
Rao, N. N., Gómezgarcía, M. R., & Kornberg, A. (2009). Inorganic polyphosphate: Essential for growth and survival. Annual Review of Biochemistry, 78, 605–647.
Ahn, K., & Kornberg, A. (1990). Polyphosphate kinase from Escherichia coli. Purification and demonstration of a phosphoenzyme intermediate. Journal of Biological Chemistry, 265(20), 11734–11739.
Parnell, A. E., Mordhorst, S., Kemper, F., Giurrandino, M., Prince, J. P., Schwarzer, N. J., Hofer, A., Wohlwend, D., Jessen, H. J., Gerhardt, S., Einsle, O., Oyston, P. C. F., Andexer, J. N., & Roach, P. L. (2018). Substrate recognition and mechanism revealed by ligand-bound polyphosphate kinase 2 structures. Proceedings of the National Academy of Sciences of the United States of America, 115(13), 3350–3355.
Nocek, B., Kochinyan, S., Proudfoot, M., Brown, G., Evdokimova, E., Osipiuk, J., Edwards, A. M., Savchenko, A., Joachimiak, A., & Yakunin, A. F. (2008). Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria. Proceedings of the National Academy of Sciences of the United States of America, 105(46), 17730–17735.
Ishige, K., Zhang, H. Y., & Arthur, K. (2002). Polyphosphate kinase (PPK2), a potent, polyphosphate-driven generator of GTP. Proceedings of the National Academy of Sciences of the United States of America, 99(26), 16684–16688.
Nocek, B. P., Khusnutdinova, A. N., Ruszkowski, M., Flick, R., Burda, M., Batyrova, K., Brown, G., Mucha, A., Joachimiak, A., Berlicki, L., & Yakunin, A. F. (2018). Structural insights into substrate selectivity and activity of bacterial polyphosphate kinases. ACS Catalysis, 8, 10746–10760.
Motomura, K., Hirota, R., Okada, M., Ikeda, T., Ishida, T., & Kuroda, A. (2014). A new subfamily of polyphosphate kinase 2 (class III PPK2) catalyzes both nucleoside monophosphate phosphorylation and nucleoside diphosphate phosphorylation. Applied and Environmental Microbiology, 80(8), 2602–2608.
Noguchi, T., & Shiba, T. (1998). Use of Escherichia coli polyphosphate kinase for oligosaccharide synthesis. Bioscience, Biotechnology, and Biochemistry, 62(8), 1594–1596.
Iwamoto, S., Motomura, K., Shinoda, Y., Urata, M., Kato, J., Takiguchi, N., Ohtake, H., Hirota, R., & Kuroda, A. (2007). Use of an Escherichia coli recombinant producing thermostable polyphosphate kinase as an ATP regenerator to produce fructose 1,6-diphosphate. Applied and Environmental Microbiology, 73(17), 5676–5678.
Sato, M., Masuda, Y., Kirimura, K., & Kino, K. (2007). Thermostable ATP regeneration system using polyphosphate kinase from Thermosynechococcus elongatus BP-1 for D-amino acid dipeptide synthesis. Journal of Bioscience and Bioengineering, 103(2), 179–184.
Mordhorst, S., Siegrist, J., Muller, M., Richter, M., & Andexer, J. N. (2017). Catalytic alkylation using a cyclic S-adenosylmethionine regeneration system. Angewandte Chemie, International Edition, 56(14), 4037–4041.
Suzuki, S., Hara, R., & Kino, K. (2018). Production of aminoacyl prolines using the adenylation domain of nonribosomal peptide synthetase with class III polyphosphate kinase 2-mediated ATP regeneration. Journal of Bioscience and Bioengineering, 125(6), 644–648.
Kulmer, S. T., Gutmann, A., Lemmerer, M., & Nidetzky, B. (2017). Biocatalytic cascade of polyphosphate kinase and sucrose synthase for synthesis of nucleotide-activated derivatives of glucose. Advanced Synthesis and Catalysis, 359(2), 292–301.
Schmacht, M., Lorenz, E., & Senz, M. (2017). Microbial production of glutathione. World Journal of Microbiology and Biotechnology, 33, 106.
Li, W., Li, Z., Yang, J., & Ye, Q. (2011). Production of glutathione using a bifunctional enzyme encoded by gshF from Streptococcus thermophilus expressed in Escherichia coli. Journal of Biotechnology, 154(4), 261–268.
Zhang, X., Wu, H., Huang, B., Li, Z. M., & Ye, Q. (2017). One-pot synthesis of glutathione by a two-enzyme cascade using a thermophilic ATP regeneration system. Journal of Biotechnology, 241, 163–169.
Batten, L. E., Parnell, A. E., Wells, N. J., Murch, A. L., Oyston, P. C. F., & Roach, P. L. (2015). Biochemical and structural characterization of polyphosphate kinase 2 from the intracellular pathogen Francisella tularensis. Bioscience Reports, 36, e00294.
Akiyama, M., Crooke, E., & Kornberg, A. (1992). The polyphosphate kinase gene of Escherichia coli. Isolation and sequence of the ppk gene and membrane location of the protein. Journal of Biological Chemistry, 267(31), 22556–22561.
Shum, K. T., Lui, E. L. H., Wong, S. C. K., Yeung, P., Sam, L., Wang, Y., Watt, R. M., & Tanner, J. A. (2011). Aptamer-mediated inhibition of Mycobacterium tuberculosis polyphosphate kinase 2. Biochemistry, 50(15), 3261–3271.
Cao, H., Nie, K., Li, C., Xu, H., Wang, F., Tan, T., & Liu, L. (2017). Rational design of substrate binding pockets in polyphosphate kinase for use in cost-effective ATP-dependent cascade reactions. Applied Microbiology and Biotechnology, 101(13), 5325–5332.
Funding
This work was supported by the China National Key Research and Development Program (Grant No. 2019YFA0904300), Shanghai Committee of Science and Technology (Grant No. 13DZ1930202) and the Fundamental Research Funds for the Central Universities (22221818014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM1
(DOC 454 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Cui, X. & Li, Z. Characterization of Two Polyphosphate Kinase 2 Enzymes Used for ATP Synthesis. Appl Biochem Biotechnol 191, 881–892 (2020). https://doi.org/10.1007/s12010-019-03224-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03224-6