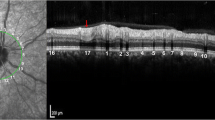
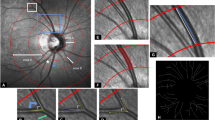
Abstract
Purpose
Preliminary to evaluate geometric indices (vessel sphericity and cylindricity) for volume-rendered optical coherence tomography angiography (OCTA) in healthy and diabetic eyes.
Methods
Twenty-six eyes of 13 healthy subjects and 12 eyes of patients with central ischemic, non-proliferative diabetic retinopathy were included. OCTA volume and surface area of the foveal vessels were measured and compared to determine OCTA sphericity and cylindricity indices and surface efficiency (SE).
Results
The overall average OCTA volume in healthy was 0.49 ± 0.09 mm3 (standard deviation [SD]), compared to 0.44 ± 0.07 mm3 (SD) in the diabetic eyes (difference in means 0.06 mm3, p = 0.054). The overall average OCTA surface area in the healthy eyes was 87.731 ± 9.51 mm2 (SD), compared to 76.65 ± 13.67 mm2 (SD) in the diabetic eyes (difference in means 11.08 mm2, p = 0.021). In relation to total foveolar tissue volume, the proportion of blood vessels was 22% in healthy individuals and only 20% in diabetics. The difference between the groups was more pronounced with respect to the total OCTA surface area, with a decrease of 13% in diabetics. A diabetic eye was most likely using geometric vessel indices analysis if the sphericity value was ≥ 0.190, with a cylindricity factor of ≥ 0.001. Reproducibility of the method was good.
Conclusions
A method for OCTA surface area and volume measurements was developed. The application of the novel OCTA sphericity and cylindricity indices could be suitable as temporal biomarker to characterize stable disease or disease progression and may contribute to a better understanding in the evolution of diabetic retinopathy.





Similar content being viewed by others
References
Wang RK, An L, Francis P, Wilson DJ (2010) Depth-resolved imaging of capillary networks in retina and choroid using ultrahigh sensitive optical microangiography. Opt Lett 35(9):1467–1469. https://doi.org/10.1364/ol.35.001467
Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A (2007) Three dimensional optical angiography. Opt Express 15(7):4083–4097
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA ophthalmology 133(1):45–50. https://doi.org/10.1001/jamaophthalmol.2014.3616
Coscas F, Sellam A, Glacet-Bernard A, Jung C, Goudot M, Miere A, Souied EH (2016) Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Investigative ophthalmology & visual science 57(9):Oct211-223. https://doi.org/10.1167/iovs.15-18793
Bradley PD, Sim DA, Keane PA, Cardoso J, Agrawal R, Tufail A, Egan CA (2016) The evaluation of diabetic macular ischemia using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57(2):626–631. https://doi.org/10.1167/iovs.15-18034
Hwang TS, Gao SS, Liu L, Lauer AK, Bailey ST, Flaxel CJ, Wilson DJ, Huang D, Jia Y (2016) Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol 134(4):367–373. https://doi.org/10.1001/jamaophthalmol.2015.5658
Al-Sheikh M, Akil H, Pfau M, Sadda SR (2016) Swept-source OCT angiography imaging of the foveal avascular zone and macular capillary network density in diabetic retinopathy. Invest Ophthalmol Vis Sci 57(8):3907–3913. https://doi.org/10.1167/iovs.16-19570
Johannesen SK, Viken JN, Vergmann AS, Grauslund J (2019) Optical coherence tomography angiography and microvascular changes in diabetic retinopathy: a systematic review. Acta Ophthalmol 97(1):7–14. https://doi.org/10.1111/aos.13859
Agemy SA, Scripsema NK, Shah CM, Chui T, Garcia PM, Lee JG, Gentile RC, Hsiao YS, Zhou Q, Ko T, Rosen RB (2015) Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina (Philadelphia, Pa) 35(11):2353–2363. https://doi.org/10.1097/iae.0000000000000862
Spaide RF (2015) Volume-rendered angiographic and structural optical coherence tomography. Retina (Philadelphia, Pa) 35(11):2181–2187. https://doi.org/10.1097/iae.0000000000000764
Spaide RF (2015) Volume-rendered optical coherence tomography of diabetic retinopathy pilot study. Am J Ophthalmol 160(6):1200–1210. https://doi.org/10.1016/j.ajo.2015.09.010
Izumo M, Lancellotti P, Suzuki K, Kou S, Shimozato T, Hayashi A, Akashi YJ, Osada N, Omiya K, Nobuoka S, Ohtaki E, Miyake F (2009) Three-dimensional echocardiographic assessments of exercise-induced changes in left ventricular shape and dyssynchrony in patients with dynamic functional mitral regurgitation. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology 10(8):961–967. https://doi.org/10.1093/ejechocard/jep114
Grande Gutierrez N, Shirinsky O, Gagarina N, Lyskina G, Fukazawa R, Ogawa S, Burns JC, Marsden AL, Kahn AM (2017) Assessment of coronary artery aneurysms caused by Kawasaki disease using transluminal attenuation gradient analysis of computerized tomography angiograms. Am J Cardiol 120(4):556–562. https://doi.org/10.1016/j.amjcard.2017.05.025
Talu S, Stach S, Calugaru DM, Lupascu CA, Nicoara SD (2017) Analysis of normal human retinal vascular network architecture using multifractal geometry. Int J Ophthalmol 10(3):434–438. https://doi.org/10.18240/ijo.2017.03.17
Talu S, Calugaru DM, Lupascu CA (2015) Characterisation of human non-proliferative diabetic retinopathy using the fractal analysis. Int J Ophthalmol 8(4):770–776. https://doi.org/10.3980/j.issn.2222-3959.2015.04.23
Stanton AV, Wasan B, Cerutti A, Ford S, Marsh R, Sever PP, Thom SA, Hughes AD (1995) Vascular network changes in the retina with age and hypertension. J Hypertens 13(12 Pt 2):1724–1728
Le D, Alam M, Miao BA, Lim JI, Yao X (2019) Fully automated geometric feature analysis in optical coherence tomography angiography for objective classification of diabetic retinopathy. Biomed Optics Express 10(5):2493–2503. https://doi.org/10.1364/boe.10.002493
Chen Z, Huang D, Izatt JA, Wang RK, Werner JS, Yasuno Y (2016) Re: Spaide et al.: volume-rendering optical coherence tomography angiography of macular telangiectasia type 2 (ophthalmology 2015;122:2261-9). Ophthalmology 123(3):e24. https://doi.org/10.1016/j.ophtha.2015.10.013
Spaide RF (2016) Volume-rendered optical coherence tomography of retinal vein occlusion pilot study. Am J Ophthalmol 165:133–144. https://doi.org/10.1016/j.ajo.2016.02.037
Maloca P, Gyger C, Schoetzau A, Hasler PW (2015) Ultra-short-term reproducibility of speckle-noise freed fluid and tissue compartmentalization of the choroid analyzed by standard OCT. Transl Vision Sci Technol 4(6):3. https://doi.org/10.1167/tvst.4.6.3
Sull AC, Vuong LN, Price LL, Srinivasan VJ, Gorczynska I, Fujimoto JG, Schuman JS, Duker JS (2010) Comparison of spectral/Fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina (Philadelphia, Pa) 30(2):235–245. https://doi.org/10.1097/IAE.0b013e3181bd2c3b
Ploner SB, Moult EM, Choi W, Waheed NK, Lee B, Novais EA, Cole ED, Potsaid B, Husvogt L, Schottenhamml J, Maier A, Rosenfeld PJ, Duker JS, Hornegger J, Fujimoto JG (2016) Toward quantitative optical coherence tomography angiography: visualizing blood flow speeds in ocular pathology using variable interscan time analysis. Retina (Philadelphia, pa) 36(Suppl 1):S118–S126. https://doi.org/10.1097/iae.0000000000001328
Bernucci MT, Merkle CW, Srinivasan VJ (2018) Investigation of artifacts in retinal and choroidal OCT angiography with a contrast agent. Biomed Optics Express 9(3):1020–1040. https://doi.org/10.1364/boe.9.001020
Fouad A, Pfefer TJ, Chen CW, Gong W, Agrawal A, Tomlins PH, Woolliams PD, Drezek RA, Chen Y (2014) Variations in optical coherence tomography resolution and uniformity: a multi-system performance comparison. Biomedical optics express 5(7):2066–2081. https://doi.org/10.1364/boe.5.002066
Spaide RF, Fujimoto JG, Waheed NK (2015) Image artifacts in optical coherence tomography angiography. Retina (Philadelphia, Pa) 35(11):2163–2180. https://doi.org/10.1097/iae.0000000000000765
Spaide RF, Fujimoto JG, Waheed NK (2015) Optical coherence tomography angiography. Retina (Philadelphia, Pa) 35(11):2161–2162. https://doi.org/10.1097/iae.0000000000000881
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G (2017) Optical coherence tomography angiography. Prog Retin Eye Res. https://doi.org/10.1016/j.preteyeres.2017.11.003
Zuiderveld K (1994) Contrast limited adaptive histograph equalization, vol 1994. Graphic Gems IV San Diego: Academic Press Professional, pp 474–485
Frangi AF, Niessen WJ, Vincken KL, Viergever MA Multiscale vessel enhancement filtering. In: Berlin, Heidelberg, 1998. Medical image computing and computer-assisted intervention — MICCAI’98. Springer, Berlin Heidelberg, pp 130–137
Kagemann L, Wollstein G, Ishikawa H, Sigal IA, Folio LS, Xu J, Gong H, Schuman JS (2011) 3D visualization of aqueous humor outflow structures in-situ in humans. Exp Eye Res 93(3):308–315. https://doi.org/10.1016/j.exer.2011.03.019
Maloca PM, Spaide RF, Rothenbuehler S, Scholl HPN, Heeren T, Ramos de Carvalho JE, Okada M, Hasler PW, Egan C, Tufail A (2019) Enhanced resolution and speckle-free three-dimensional printing of macular optical coherence tomography angiography. Acta Ophthalmol 97(2):e317–e319. https://doi.org/10.1111/aos.13567
Maloca PM, Tufail A, Hasler PW, Rothenbuehler S, Egan C, Ramos de Carvalho JE, Spaide RF (2019) 3D printing of the choroidal vessels and tumours based on optical coherence tomography. Acta Ophthalmol 97(2):e313–e316. https://doi.org/10.1111/aos.13637
Kassab GS (2006) Biomechanics of the cardiovascular system: the aorta as an illustratory example. J R Soc Interface 3(11):719–740. https://doi.org/10.1098/rsif.2006.0138
Emami A (06/2014) 360 ° Industrial Design Grundlagen der analytischen Produktgestaltung
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6(4):284–290. https://doi.org/10.1037/1040-3590.6.4.284
Ishibazawa A, Nagaoka T, Takahashi A, Omae T, Tani T, Sogawa K, Yokota H, Yoshida A (2015) Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol 160(1):35-44.e31. https://doi.org/10.1016/j.ajo.2015.04.021
Samara WA, Shahlaee A, Adam MK, Khan MA, Chiang A, Maguire JI, Hsu J, Ho AC (2017) Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology 124(2):235–244. https://doi.org/10.1016/j.ophtha.2016.10.008
Hwang TS, Jia Y, Gao SS, Bailey ST, Lauer AK, Flaxel CJ, Wilson DJ, Huang D (2015) Optical coherence tomography angiography features of diabetic retinopathy. Retina (Philadelphia, Pa) 35(11):2371–2376. https://doi.org/10.1097/iae.0000000000000716
Di G, Weihong Y, Xiao Z, Zhikun Y, Xuan Z, Yi Q, Fangtian D (2016) A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 254(5):873–879. https://doi.org/10.1007/s00417-015-3143-7
Couturier A, Mane V, Bonnin S, Erginay A, Massin P, Gaudric A, Tadayoni R (2015) Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina (Philadelphia, Pa) 35(11):2384–2391. https://doi.org/10.1097/iae.0000000000000859
Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH (2016) Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Investigative ophthalmology & visual science 57(9):Oct362-370. https://doi.org/10.1167/iovs.15-18904
Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y (2016) Automated quantification of nonperfusion in three retinal plexuses using projection-resolved optical coherence tomography angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci 57(13):5101–5106. https://doi.org/10.1167/iovs.16-19776
Dimitrova G, Chihara E, Takahashi H, Amano H, Okazaki K (2017) Author response: quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Invest Ophthalmol Vis Sci 58(3):1767. https://doi.org/10.1167/iovs.17-21706
Cao D, Yang D, Huang Z, Zeng Y, Wang J, Hu Y, Zhang L (2018) Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. https://doi.org/10.1007/s00592-018-1115-1
An D, Balaratnasingam C, Heisler M, Francke A, Ju M, McAllister IL, Sarunic M, Yu DY (2018) Quantitative comparisons between optical coherence tomography angiography and matched histology in the human eye. Exp Eye Res 170:13–19. https://doi.org/10.1016/j.exer.2018.02.006
Tan PE, Balaratnasingam C, Xu J, Mammo Z, Han SX, Mackenzie P, Kirker AW, Albiani D, Merkur AB, Sarunic MV, Yu DY (2015) Quantitative comparison of retinal capillary images derived by speckle variance optical coherence tomography with histology. Invest Ophthalmol Vis Sci 56(6):3989–3996. https://doi.org/10.1167/iovs.14-15879
Dong J, Jia YD, Wu Q, Zhang S, Jia Y, Huang D, Wang X (2017) Interchangeability and reliability of macular perfusion parameter measurements using optical coherence tomography angiography. Br J Ophthalmol 101(11):1542–1549. https://doi.org/10.1136/bjophthalmol-2016-309441
Guo J, She X, Liu X, Sun X (2017) Repeatability and reproducibility of foveal avascular zone area measurements using AngioPlex spectral domain optical coherence tomography angiography in healthy subjects. Ophthalmologica Journal international d'ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde 237(1):21–28. https://doi.org/10.1159/000453112
Carpineto P, Mastropasqua R, Marchini G, Toto L, Di Nicola M, Di Antonio L (2016) Reproducibility and repeatability of foveal avascular zone measurements in healthy subjects by optical coherence tomography angiography. Br J Ophthalmol 100(5):671–676. https://doi.org/10.1136/bjophthalmol-2015-307330
You Q, Freeman WR, Weinreb RN, Zangwill L, Manalastas PIC, Saunders LJ, Nudleman E (2017) Reproducibility of vessel density measurement with optical coherence tomography angiography in eyes with and without retinopathy. Retina (Philadelphia, Pa) 37(8):1475–1482. https://doi.org/10.1097/iae.0000000000001407
Shiihara H, Sakamoto T, Yamashita T, Kakiuchi N, Otsuka H, Terasaki H, Sonoda S (2017) Reproducibility and differences in area of foveal avascular zone measured by three different optical coherence tomographic angiography instruments. Sci Rep 7(1):9853. https://doi.org/10.1038/s41598-017-09255-5
Krawitz BD, Mo S, Geyman LS, Agemy SA, Scripsema NK, Garcia PM, Chui TY, Rosen RB (2017) Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vis Res. https://doi.org/10.1016/j.visres.2016.09.019
Goudot MM, Sikorav A, Semoun O, Miere A, Jung C, Courbebaisse B, Srour M, Freiha JG, Souied EH (2017) Parafoveal OCT angiography features in diabetic patients without clinical diabetic retinopathy: a qualitative and quantitative analysis. J Ophthalmol 2017:8676091. https://doi.org/10.1155/2017/8676091
Rolfe DF, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77(3):731–758
Geiser MH, Bonvin M, Quibel O (2004) Corneal and retinal temperatures under various ambient conditions: a model and experimental approach. Klinische Monatsblatter fur Augenheilkunde 221(5):311–314. https://doi.org/10.1055/s-2004-812878
Meiri S (2011) Bergmann's Rule – what's in a name? Global Ecology and Biogeography 20(1 January 2011):203–207. https://doi.org/10.1111/j.1466-8238.2010.00577.x
James H, Brown AKL (1969) Bergmann's rule and climatic adaptation in Woodrats (Neotoma). Evolution 23(2):329–338. https://doi.org/10.2307/2406795
Iafe NA, Phasukkijwatana N, Chen X, Sarraf D (2016) Retinal capillary density and Foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57(13):5780–5787. https://doi.org/10.1167/iovs.16-20045
Wong TY, Shankar A, Klein R, Klein BE, Hubbard LD (2004) Prospective cohort study of retinal vessel diameters and risk of hypertension. BMJ (Clinical research ed) 329(7457):79. https://doi.org/10.1136/bmj.38124.682523.55
Leung H, Wang JJ, Rochtchina E, Tan AG, Wong TY, Klein R, Hubbard LD, Mitchell P (2003) Relationships between age, blood pressure, and retinal vessel diameters in an older population. Invest Ophthalmol Vis Sci 44(7):2900–2904
Li J, Yang YQ, Yang DY, Liu XX, Sun YX, Wei SF, Wang NL (2016) Reproducibility of perfusion parameters of optic disc and macula in rhesus monkeys by optical coherence tomography angiography. Chin Med J 129(9):1087–1090. https://doi.org/10.4103/0366-6999.180532
Faulkner L (2003) Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc 35(3):379–383
Spaide RF, Curcio CA (2017) Evaluation of segmentation of the superficial and deep vascular layers of the retina by optical coherence tomography angiography instruments in Normal eyes. JAMA ophthalmology 135(3):259–262. https://doi.org/10.1001/jamaophthalmol.2016.5327
Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, Shi Y, Wang RK (2017) Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res 60:66–100. https://doi.org/10.1016/j.preteyeres.2017.07.002
Baran U, Choi WJ, Li Y, Wang RK (2017) Tail artifact removal in OCT angiography images of rodent cortex. J Biophotonics 10(11):1421–1429. https://doi.org/10.1002/jbio.201600194
Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G (2018) Optical coherence tomography angiography. Prog Retin Eye Res 64:1–55. https://doi.org/10.1016/j.preteyeres.2017.11.003
Zhang M, Hwang TS, Campbell JP, Bailey ST, Wilson DJ, Huang D, Jia Y (2016) Projection-resolved optical coherence tomographic angiography. Biomedical optics express 7(3):816–828. https://doi.org/10.1364/boe.7.000816
Liu L, Gao SS, Bailey ST, Huang D, Li D, Jia Y (2015) Automated choroidal neovascularization detection algorithm for optical coherence tomography angiography. Biomedical optics express 6(9):3564–3576. https://doi.org/10.1364/boe.6.003564
Li P, Huang Z, Yang S, Liu X, Ren Q, Li P (2017) Adaptive classifier allows enhanced flow contrast in OCT angiography using a histogram-based motion threshold and 3D hessian analysis-based shape filtering. Opt Lett 42(23):4816–4819. https://doi.org/10.1364/ol.42.004816
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Peter Maloca is a consultant at ZEISS FORUM. Catherine Egan and Adnan Tufail received a financial grant from the National Institute for Health Research (NIHR) Biomedical Research Centre, based at Moorfields Eye Hospital, and also from the NHS Foundation Trust and the UCL Institute of Ophthalmology. Adnan Tufail is employed is a consultant for Heidelberg Engineering and Optovue. Richard Spaide and Peter Maloca are consultant for Topcon. Richard Spaide holds the patent for Frangi vessel segmentation. The funding organizations had no role in the design or conduct of the current study. The views expressed are those of the authors and not necessarily those of the National Eye Institute, the NHS, the NIHR, or the Department of Health.
Dr. Hendrik Scholl is supported by the Foundation Fighting Blindness Clinical Research Institute (FFB CRI); Shulsky Foundation, New York, NY; National Centre of Competence in Research (NCCR) Molecular Systems Engineering (University of Basel and ETH Zürich); Swiss National Science Foundation; and Wellcome Trust.
Dr. Scholl is a paid consultant of the following entities: Boehringer Ingelheim Pharma GmbH & Co. KG; Gerson Lehrman Group; and Guidepoint.
Dr. Scholl is member of the Scientific Advisory Board of the Astellas Institute for Regenerative Medicine; Gensight Biologics; Intellia Therapeutics, Inc.; Ionis Pharmaceuticals, Inc.; ReNeuron Group Plc/Ora Inc.; Pharma Research & Early Development (pRED) of F. Hoffmann-La Roche Ltd.; and Vision Medicines, Inc.
Dr. Scholl is member of the Data Monitoring and Safety Board/Committee of the following entities: Genentech Inc./F. Hoffmann-La Roche Ltd. and ReNeuron Group Plc/Ora Inc. Dr. Scholl is member of the steering committee of the following entities: Novo Nordisk (FOCUS trial).
Dr. Scholl is co-director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB) which is constituted as a nonprofit foundation and receives funding from the University of Basel, the University Hospital Basel, Novartis, and the government of Basel-Stadt.
These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Johns Hopkins University and Bayer Pharma AG have an active research collaboration and option agreement. These arrangements have also been reviewed and approved by the University of Basel (Universitätsspital Basel, USB) in accordance with its conflict of interest policies.
Dr. Hendrik Scholl is principal investigator of grants at the USB sponsored by the following entity: Acucela Inc.; Aegerion Pharmaceuticals (Novelion Therapeutics); Kinarus AG; NightstaRx Ltd.; Ophthotech Corporation; and Spark Therapeutics England, Ltd. Grants at USB are negotiated and administered by the institution (USB) which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s).
Others: None. This does not alter our adherence to Graefe’s policies on sharing data and materials. The mentioned institutions had no role in the design or conduct of this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maloca, P.M., Spaide, R.F., de Carvalho, E.R. et al. Novel biomarker of sphericity and cylindricity indices in volume-rendering optical coherence tomography angiography in normal and diabetic eyes: a preliminary study. Graefes Arch Clin Exp Ophthalmol 258, 711–723 (2020). https://doi.org/10.1007/s00417-019-04582-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04582-x