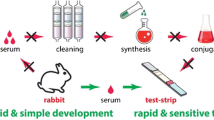
Abstract
We produced a prometryn-specific monoclonal antibody and propose a strategy for convenient on-site detection of prometryn residues in herbs for the first time. This strategy has perfect applicability in a complex herbal medicine matrix. The strategy combines a semiquantitative immunochromatographic strip assay with a heterologous indirect competitive ELISA. When there was no matrix interference, the ELISA had a half-maximal inhibitory concentration of 2.6 ng·mL-1 and a limit of detection of 0.2 ng·mL-1. The immunochromatographic strip assay can be completed within 5 min with a visual limit of detection of 1 ng·mL-1. Although the sample matrix had different effects on the sensitivity of the antibody, excellent repeatability and accuracy were achieved. The method was successfully applied for the screening and determination of prometryn residue in multiple complex herb samples for the first time, and the results were in good agreement with those obtained by liquid chromatography–tandem mass spectrometry. The proposed strategy is rapid, of high-throughput, and of low cost, and may be a promising choice for on-site detection of prometryn in different kinds of herbs.

Graphical abstract







Similar content being viewed by others
References
Heri W, Pfister F, Carroll B, Parshley T, Nabors JB. Production, development, and registration of triazine herbicides. In: LeBaron HM, McFarland JE, Burnside OC, editors. The Triazine Herbicides. Elsevier; 2008. 50:31–43. https://doi.org/10.1016/B978-044451167-6.50006-4.
Guo LJ, Qu JR, Miao SS, Geng HR, Yang H. Development of a molecularly imprinted polymer for prometryne clean-up in the environment. J Sep Sci. 2014;36(24):3911–7.
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E. A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf. 2015;116:1–9.
Ren C, Tian X, Sun Y, Deng X, Liu H, Xue J, et al. Residues and risk assessment of 13 triazine herbicides in Apostichopus japonicus. Modern Food Science and Technology. 2014;30(3):244–9.
Velisek J, Stara A, Koutnik D, Machova J. Effects of prometryne on early life stages of common carp (Cyprinus carpio L.). Pestic Biochem Physiol. 2015;118:58–63.
Wang Y, Zhang G, Wang L. Interaction of prometryn to human serum albumin: insights from spectroscopic and molecular docking studies. Pestic Biochem Physiol. 2014;108:66–73.
PAN Pesticides Database. Pesticide Action Network North America http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC34259.
Zhou J, Hu F, Jiao J, Liu M, Li H. Effects of bacterial-feeding nematodes and prometryne-degrading bacteria on the dissipation of prometryne in contaminated soil. J Soil Sediment. 2012;12(4):576–85.
Vryzas Z, Alexoudis C, Vassiliou G, Galanis K, Papadopoulou-Mourkidou E. Determination and aquatic risk assessment of pesticide residues in riparian drainage canals in northeastern Greece. Ecotoxicol Environ Saf. 2011;74(2):174–81.
Caquet T, Roucaute M, Mazzella N, Delmas F, Madigou C, Farcy E, et al. Risk assessment of herbicides and booster biocides along estuarine continuums in the Bay of Vilaine area (Brittany, France). Environ Sci Pollut Res. 2013;20(2):651–66.
Ministry of Health, Labour and Welfate, Japan (Enforcement on May 29, 2006) Positive list system for agricultural chemical residues in foods. http://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228.
G/SPS/N/CAN/671 (2013) Proposed maximum residue limit: prometryn (PMRL2013-16).
G/SPS/N/USA/2585 (2013) Prometryn; pesticide tolerances. World Trade Organization, Committee on Sanitary and Phytosanitary Measures.
GB2763-2016 National food safety standard - maximum residue limits for pesticides in food.
Code of Federal Regulations, Part 180 - tolerances and exemptions for pesticide chemical residues in food. US Environmental Protection Agency.
Wang L, Wang L, Wu M. The screening of suitable herbicide for Angelica. Journal of Agricultural Science Yanbian University. 2008;30(1):46–51.
Wang L, Wang L, Wu M. Analysis of residual prometryme in Angelica. Journal of Agricultural Science Yanbian University. 2007;29(4):262–6.
Zhou J, Chen J, Cheng Y, Li D, Hu F, Li H. Determination of prometryne in water and soil by HPLC–UV using cloud-point extraction. Talanta. 2009;79(2):189–93.
Wu M, Li T, Zheng C. Analysis of prometryn residue in roots of Codonopsis lanceolata. Plant Prot. 2010;36(6):100–2.
Chae Y, Cho Y, Jang K, Kim J, Lee S, Chang M. Establishment of an analytical method for prometryn residues in clam using GC-MS. Korean J Food Sci Technol. 2013;45(5):531–6.
Sun S, Li Y, Lv P, Punamiya P, Sarkar D, Dan Y, et al. Determination of prometryn in vetiver grass and water using gas chromatography–nitrogen chemiluminescence detection. J Chromatogr Sci. 2016;54(2):97–102.
Kramer K. Synthesis of a group-selective antibody library against haptens. J Immunol Methods. 2002;266(1):209–20.
Na Y, Sheng W, Yuan M, Li L, Liu B, Zhang Y, et al. Enzyme-linked immunosorbent assay and immunochromatographic strip for rapid detection of atrazine in water samples. Microchim Acta. 2012;177(1-2):177–84.
Delaunay-Bertoncini N, Pichon V, Hennion M-C. Experimental comparison of three monoclonal antibodies for the class-selective immunoextraction of triazines. J Chromatogr A. 2003;999(1-2):3–15.
Liu C, Dou X, Lei Z, Kong W, Liu W, Duan Y, et al. Development of a broad-specificity antibody-based immunoassay for triazines in ginger and the quantitative structure-activity relationship study of cross-reactive molecules by molecular modeling. Anal Chim Acta. 2018;1012:90–9.
Wang Y, Wei D, Yang H, Yang Y, Xing W, Li Y, et al. Development of a highly sensitive and specific monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of Sudan I in food samples. Talanta. 2009;77(5):1783–9.
Cui Y, Nan T, Tan G, Li QX, Wang B, Liu S. Production of monoclonal antibody to herbicide fenoxaprop-ethyl. Hybridoma. 2011;30(5):463–7.
Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987;100(1-2):173–9.
Cui Y, Liu K, Xu C, Liu F, Li QX, Liu S, et al. Development of a sensitive monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for analysing chlorantraniliprole residues. Food Chem. 2014;143:293–9.
Zhou W, Kong W, Dou X, Zhao M, Ouyang Z, Yang M. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J Chromatogr B. 2016;1022:102–8.
Chen X, Liu L, Kuang H, Song S, Xu C. A strip-based immunoassay for rapid determination of fenpropathrin. Anal Methods. 2013;5(21):6234–9.
Liu C, Dou X, Zhang L, Li Q, Qin J, Duan Y, et al. Determination of triazine herbicides and their metabolites in multiple medicinal parts of traditional Chinese medicines using streamlined pretreatment and UFLC-ESI-MS/MS. Chemosphere. 2017;190:103–13.
Zhang B, Nan T, Zhan Z, Kang L, Yang J, Yuan Y, et al. Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for luteoloside detection in Flos Lonicerae Japonicae. Anal Bioanal Chem. 2016;408(22):6053–61.
Sapozhnikova Y, Simons T, Lehotay SJ. Evaluation of a fast and simple sample preparation method for polybrominated diphenyl ether (PBDE) flame retardants and dichlorodiphenyltrichloroethane (DDT) pesticides in fish for analysis by ELISA compared with GC-MS/MS. J Agric Food Chem. 2015;63(18):4429–34.
Qian G, Wang L, Wu Y, Zhang Q, Sun Q, Liu Y, et al. A monoclonal antibody-based sensitive enzyme-linked immunosorbent assay (ELISA) for the analysis of the organophosphorous pesticides chlorpyrifos-methyl in real samples. Food chem. 2009;117(2):364–70.
Geis-Asteggiante L, Lehotay SJ, Fortis LL, et al. Development and validation of a rapid method for microcystins in fish and comparing LC-MS/MS results with ELISA. Anal Bioanal Chem. 2011;401(8):2617–30.
European Commission. Guidance document on analytical quality control and method validation procedures for pesticides residues analysis in food and feed. 2015. SANTE/11945/2015.
Gascón J, Oubina A, Ballesteros B, Barceló D, Camps F, Marco M-P, et al. Development of a highly sensitive enzyme-linked immunosorbent assay for atrazine Performance evaluation by flow injection immunoassay. Anal Chim Acta. 1997;347:149–62.
Sai N, Sun W, Wu Y, Sun Z, Yu G, Huang G. A highly sensitive immunoassay for atrazine based on covalently linking the small molecule hapten to a urea–glutaraldehyde network on a polystyrene surface. Int Immunopharmacol. 2016;40:480–6.
Bruun L, Koch C, Jakobsen MH, Aamand J. New monoclonal antibody for the sensitive detection of hydroxy-s-triazines in water by enzyme-linked immunosorbent assay. Anal Chim Acta. 2000;423(2):205–13.
Maqbool U, Haq A-U, Qureshi MJ, Iqbal MZ, Hock B, Kramer K. Development of ELISA technique for analysis of atrazine residues in water. J Environ Sci Health B. 2002;37(4):307–22.
Wortberg M, Goodrow MH, Gee SJ, Hammock BD. Immunoassay for simazine and atrazine with low cross-reactivity for propazine. J Agric Food Chem. 1996;44(8):2210–9.
Gabaldón JA, Maquieira A, Puchades R. Determination of atrazine in vegetable samples using a dipstick immunoassay format. Int J Environ Anal Chem. 2002;82(3):145–55.
Wittmann C, Bilitewski U, Giersch T, Kettling U, Schmid RD. Development and evaluation of a dipstick immunoassay format for the determination of atrazine residues on-site. Analyst. 1996;121(6):863–9.
Turiel E, Fernández P, Pérez-Conde C, Gutiérrez AM, Cámara C. Flow-through fluorescence immunosensor for atrazine determination. Talanta. 1998;47(5):1255–61.
Gonzalez-Techera A, Zon MA, Molina PG, Fernandez H, Gonzalez-Sapienza G, Arevalo FJ. Development of a highly sensitive noncompetitive electrochemical immunosensor for the detection of atrazine by phage anti-immunocomplex assay. Biosens Bioelectron. 2015;64:650–6.
Ciumasu IM, Krämer PM, Weber CM, Kolb G, Tiemann D, Windisch S, et al. A new, versatile field immunosensor for environmental pollutants: Development and proof of principle with TNT, diuron, and atrazine. Biosens Bioelectron. 2006;21(2):354–64.
Acknowledgements
We thank Baomin Wang from the College of Agronomy and Biotechnology, China Agricultural University, China, for providing technical guidance.
Funding
This work was supported by the National Natural Science Foundation of China (no. 81573595 and 81703699), the CAMS Innovation Fund for Medical Sciences (no. 2017-I2M-1-013) and the Biology Key Construction Discipline Fund project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
The use of laboratory animals (mice) was in accordance with the relevant Chinese laws and according to the Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College regulations concerning protection of animals used for scientific purposes. The mice used in this study were approved by the Ethics Committee on Experimental Animals and Animal Tests of Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College. The review number is SLXD-20170301058.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 753 kb)
Rights and permissions
About this article
Cite this article
Liu, C., Wang, Y., Zhang, L. et al. An integrated strategy for rapid on-site screening and determination of prometryn residues in herbs. Anal Bioanal Chem 412, 621–633 (2020). https://doi.org/10.1007/s00216-019-02224-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02224-z