Abstract
Purpose
Sarcopenia has been reported to be associated with higher mortality and increased toxicity of chemotherapy in breast cancer patients. However, evidence from Asian countries is scarce. Here, we investigated the association between sarcopenia and the frequency of severe laboratory adverse events due to perioperative chemotherapy in Japanese breast cancer patients.
Methods
Eighty-two patients with breast cancer receiving perioperative epirubicin plus cyclophosphamide therapy were evaluated. Skeletal muscle of the cross-sectional area at the third lumbar vertebra was measured by computed tomography, and sarcopenia was defined as skeletal muscle index < 40 cm2/m2. Laboratory toxicity during all cycles of perioperative chemotherapy was assessed. The study endpoint was the frequency of severe (grade 3 or more) laboratory adverse events.
Results
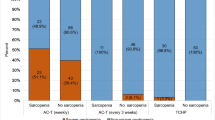
Overall, 10 patients (12.2%) were classified as sarcopenic. The frequency of severe laboratory adverse events was 28.0%, and this was significantly higher in sarcopenic patients compared to non-sarcopenic patients (70% vs. 22.2%, odds ratio 7.9 (95% CI, 1.6–52.8), p = 0.004). Neither of body weight, body mass index, area of visceral adipose tissue, subcutaneous adipose tissue, nor skeletal muscle density was significantly associated with the frequency of severe laboratory adverse events.
Conclusion
Sarcopenia was a significant risk factor of severe laboratory toxicity in breast cancer patients receiving perioperative epirubicin plus cyclophosphamide therapy. This finding raises the potential use of body composition assessment to predict the risk of chemotherapy toxicity and determine an individualized treatment strategy.

Similar content being viewed by others
References
Kwan ML, Chen WY, Kroenke CH, Weltzien EK, Beasley JM, Nechuta SJ, Poole EM, Lu W, Holmes MD, Quesenberry CP Jr, Pierce JP, Shu XO, Caan BJ (2012) Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat 132:729–739
Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T (2014) Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 25:1901–1914
Villaseñor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, Neuhouser ML (2012) Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv 6:398–406
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37:1101–1013
Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, Quesenberry CP, Weltzien EK, Castillo AL, Olobatuyi TA, Chen WY (2018) Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4:798–804
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26:861–868
Rier HN, Jager A, Sleijfer S, van Rosmalen J, Kock MCJM, Levin MD (2017) Low muscle attenuation is a prognostic factor for survival in metastatic breast cancer patients treated with first line palliative chemotherapy. Breast 31:9–15
Bozzetti F (2017) Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 28:2107–2118
Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, Koski S, Pituskin E, Sawyer MB (2009) Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res 15:2920–2926
Shachar SS, Deal AM, Weinberg M, Nyrop KA, Williams GR, Nishijima TF, Benbow JM, Muss HB (2017) Skeletal muscle measures as predictors of toxicity, hospitalization, and survival in patients with metastatic breast cancer receiving taxane-based chemotherapy. Clin Cancer Res 23:658–665
Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, Choi SK, Muss HB (2017) Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res 23:3537–3543
Mazzuca F, Onesti CE, Roberto M, Di Girolamo M, Botticelli A, Begini P et al (2018) Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget 9:25714–25722
Aleixo GFP, Williams GR, Nyrop KA, Muss HB, Shachar SS (2019) Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat 177:569–579
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y (1999) Abdominal fat: standardized technique for measurement at CT. Radiology 211:283–286
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Song EJ, Lee CW, Jung SY, Kim BN, Lee KS, Lee S, Kang HS, Park IH, Lee MH, Kim YJ, Ko K, Kim S, Nam BH, Lee ES (2018) Prognostic impact of skeletal muscle volume derived from cross-sectional computed tomography images in breast cancer. Breast Cancer Res Treat 172:425–436
Kim EY, Kim K, Kim YS, Ahn HK, Jeong YM, Kim JH, Choi WJ (2017) Prevalence of and factors associated with sarcopenia in Korean cancer survivors: based on data obtained by the Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2011. Nutr Cancer 69:394–401
Ma B, Yeo W, Hui P, Ho WM, Johnson PJ (2002) Acute toxicity of adjuvant doxorubicin and cyclophosphamide for early breast cancer -- a retrospective review of Chinese patients and comparison with an historic Western series. Radiother Oncol 62:185–189
Kenmotsu H, Tanigawara Y (2015) Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the Western dose. Cancer Sci 106:497–504
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 15:95–101
Tan BH, Brammer K, Randhawa N, Welch NT, Parsons SL, James EJ, Catton JA (2015) Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Eur J Surg Oncol 41:333–338
Prado CM, Lima IS, Baracos VE, Bies RR, McCargar LJ, Reiman T, Mackey JR, Kuzma M, Damaraju VL, Sawyer MB (2011) An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol 67:93–101
Hamaker ME, Schiphorst AH, Huinink DB, Schaar C, Munster BC (2014) The effect of a geriatric evaluation on treatment decisions for older cancer patients--a systematic review. Acta Oncol 53:289–296
Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, Canin B, Cohen HJ, Holmes HM, Hopkins JO, Janelsins MC, Khorana AA, Klepin HD, Lichtman SM, Mustian KM, Tew WP, Hurria A (2018) Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol 36:2326–2347
Jones RL, Walsh G, Ashley S, Chua S, Agarwal R, O'Brien M, Johnston S, Smith IE (2009) A randomised pilot Phase II study of doxorubicin and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC) given 2 weekly with pegfilgrastim (accelerated) vs 3 weekly (standard) for women with early breast cancer. Br J Cancer 100:305–310
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC (2017) ESPEN guidelines on nutrition in cancer patients. Clin Nutr 36:11–48
Adams SC, Segal RJ, McKenzie DC, Vallerand JR, Morielli AR, Mackey JR, Gelmon K, Friedenreich CM, Reid RD, Courneya KS (2016) Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Breast Cancer Res Treat 158:497–507
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, Spicer DV, Tripathy D, Bernstein L, Mortimer JE (2018) Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncol 36:875–883
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained in an opt-out system from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ueno, A., Yamaguchi, K., Sudo, M. et al. Sarcopenia as a risk factor of severe laboratory adverse events in breast cancer patients receiving perioperative epirubicin plus cyclophosphamide therapy. Support Care Cancer 28, 4249–4254 (2020). https://doi.org/10.1007/s00520-019-05279-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-019-05279-x