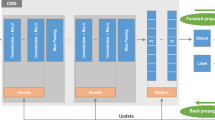
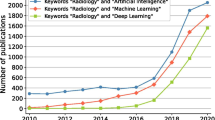
Abstract
Deep learning with convolutional neural networks (CNN) is a rapidly advancing subset of artificial intelligence that is ideally suited to solving image-based problems. There are an increasing number of musculoskeletal applications of deep learning, which can be conceptually divided into the categories of lesion detection, classification, segmentation, and non-interpretive tasks. Numerous examples of deep learning achieving expert-level performance in specific tasks in all four categories have been demonstrated in the past few years, although comprehensive interpretation of imaging examinations has not yet been achieved. It is important for the practicing musculoskeletal radiologist to understand the current scope of deep learning as it relates to musculoskeletal radiology. Interest in deep learning from researchers, radiology leadership, and industry continues to increase, and it is likely that these developments will impact the daily practice of musculoskeletal radiology in the near future.



Similar content being viewed by others
References
Choy G, Khalilzadeh O, Michalski M, Do S, Samir AE, Pianykh OS, et al. Current applications and future impact of machine learning in radiology. Radiology. 2018;288:318–28.
Soffer S, Ben-Cohen A, Shimon O, Amitai MM, Greenspan H, Klang E. Convolutional neural networks for radiologic images: a radiologist’s guide. Radiology. 2019;290:590–606.
Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, et al. Deep learning: a primer for radiologists. RadioGraphics. 2017;37:2113–31.
Kruskal JB, Berkowitz S, Geis JR, Kim W, Nagy P, Dreyer K. Big data and machine learning—strategies for driving this bus: a summary of the 2016 Intersociety Summer Conference. J Am Coll Radiol. 2017;14:811–7.
Rosenkrantz AB, Nicola GN, Allen B, Hughes DR, Hirsch JA. MACRA, MIPS, and the new Medicare quality payment program: an update for radiologists. J Am Coll Radiol. 2017;14:316–23.
Mazurowski MA. Artificial intelligence may cause a significant disruption to the radiology workforce. J Am Coll Radiol [Internet]. 2019 [cited 2019 May 4];0. Available from: https://www.jacr.org/article/S1546-1440(19)30064-X/abstract
Yamashita R, Nishio M, Do RKG, Togashi K. Convolutional neural networks: an overview and application in radiology. Insights Imaging. 2018;9:611–29.
Levine AB, Schlosser C, Grewal J, Coope R, Jones SJM, Yip S. Rise of the machines: advances in deep learning for cancer diagnosis. Trends Cancer. 2019;5:157–69.
Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, et al. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J [Internet]. [cited 2019 May 4]; Available from: https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehz056/5366208.
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O. Deep learning with convolutional neural network in radiology. Jpn J Radiol. 2018;36:257–72.
Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, et al. ImageNet Large Scale Visual Recognition Challenge. ArXiv14090575 Cs [Internet]. 2014 [cited 2019 May 4]; Available from: http://arxiv.org/abs/1409.0575.
Krizhevsky A, Sutskever I, Hinton GE. ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L, Weinberger KQ, editors. Adv Neural Inf Process Syst 25 [Internet]. Curran Associates, Inc.; 2012 [cited 2018 Nov 11]. p. 1097–1105. Available from: http://papers.nips.cc/paper/4824-imagenet-classification-with-deep-convolutional-neural-networks.pdf.
Hinton GE, Srivastava N, Krizhevsky A, Sutskever I, Salakhutdinov RR. Improving neural networks by preventing co-adaptation of feature detectors. ArXiv12070580 Cs [Internet]. 2012 [cited 2019 May 4]; Available from: http://arxiv.org/abs/1207.0580
Abadi M, Agarwal A, Barham P, Brevdo E, Chen Z, Citro C, et al. TensorFlow: Large-scale machine learning on heterogeneous distributed systems. ArXiv160304467 Cs [Internet]. 2016 [cited 2019 May 4]; Available from: http://arxiv.org/abs/1603.04467.
Home - Keras Documentation [Internet]. [cited 2019 May 4]. Available from: https://keras.io/
Caffe2 [Internet]. Caffe2. [cited 2019 May 4]. Available from: http://caffe2.ai/
PyTorch documentation — PyTorch master documentation [Internet]. [cited 2019 May 4]. Available from: https://pytorch.org/docs/stable/index.html.
Tomita N, Cheung YY, Hassanpour S. Deep neural networks for automatic detection of osteoporotic vertebral fractures on CT scans. Comput Biol Med. 2018;98:8–15.
Roth HR, Wang Y, Yao J, Lu L, Burns JE, Summers RM. Deep convolutional networks for automated detection of posterior-element fractures on spine CT. ArXiv160200020 Cs. 2016;97850P.
Olczak J, Fahlberg N, Maki A, Razavian AS, Jilert A, Stark A, et al. Artificial intelligence for analyzing orthopedic trauma radiographs: deep learning algorithms—are they on par with humans for diagnosing fractures? Acta Orthop. 2017;88:581–6.
Urakawa T, Tanaka Y, Goto S, Matsuzawa H, Watanabe K, Endo N. Detecting intertrochanteric hip fractures with orthopedist-level accuracy using a deep convolutional neural network. Skelet Radiol. 2019;48:239–44.
Rajpurkar P, Irvin J, Bagul A, Ding D, Duan T, Mehta H, et al. MURA: large dataset for abnormality detection in musculoskeletal radiographs. 2017 [cited 2019 Apr 8]; Available from: https://arxiv.org/abs/1712.06957v4.
Chung SW, Han SS, Lee JW, Oh K-S, Kim NR, Yoon JP, et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop. 2018;89:468–73.
Kim DH, MacKinnon T. Artificial intelligence in fracture detection: transfer learning from deep convolutional neural networks. Clin Radiol. 2018;73:439–45.
Lindsey R, Daluiski A, Chopra S, Lachapelle A, Mozer M, Sicular S, et al. Deep neural network improves fracture detection by clinicians. Proc Natl Acad Sci. 2018;115:11591–6.
Pranata YD. Deep learning and SURF for automated classification and detection of calcaneus fractures in CT images. Comput Methods Prog Biomed. 2019;11.
Couteaux V, Si-Mohamed S, Nempont O, Lefevre T, Popoff A, Pizaine G, et al. Automatic knee meniscus tear detection and orientation classification with Mask-RCNN. Diagn Interv Imaging. 2019.
Roblot V, Giret Y, Bou Antoun M, Morillot C, Chassin X, Cotten A, et al. Artificial intelligence to diagnose meniscus tears on MRI. Diagn Interv Imaging. 2019.
Lassau N, Estienne T, de Vomecourt P, Azoulay M, Cagnol J, Garcia G, et al. Five simultaneous artificial intelligence data challenges on ultrasound, CT, and MRI. Diagn Interv Imaging 2019.
Bien N, Rajpurkar P, Ball RL, Irvin J, Park A, Jones E, et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet. PLoS Med. 2018;15:e1002699.
Pedoia V, Norman B, Mehany SN, Bucknor MD, Link TM, Majumdar S. 3D convolutional neural networks for detection and severity staging of meniscus and PFJ cartilage morphological degenerative changes in osteoarthritis and anterior cruciate ligament subjects. J Magn Reson Imaging [Internet]. [cited 2018 Oct 22];0. Available from: http://onlinelibrary.wiley.com/doi/abs/10.1002/jmri.26246.
Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep Learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology. 2018;289:160–9.
Abidin AZ, Deng B, DSouza AM, Nagarajan MB, Coan P, Wismüller A. Deep transfer learning for characterizing chondrocyte patterns in phase contrast X-ray computed tomography images of the human patellar cartilage. Computers in Biology and Medicine. 2018;95:24–33.
Pedoia V, Lee J, Norman B, Link TM, Majumdar S. Diagnosing osteoarthritis from T2 maps using deep learning: an analysis of the entire osteoarthritis initiative baseline cohort. Osteoarthr Cartil 2019.
Chang PD, Wong TT, Rasiej MJ. Deep learning for detection of complete anterior cruciate ligament tear. J Digit Imaging [Internet]. 2019 [cited 2019 Apr 8]; Available from: http://link.springer.com/10.1007/s10278-019-00193-4.
Jamaludin A, Kadir T, Zisserman A. SpineNet: Automatically Pinpointing Classification Evidence in Spinal MRIs. In: Ourselin S, Joskowicz L, Sabuncu MR, Unal G, Wells W, editors. Med Image Comput Comput-Assist Interv – MICCAI 2016 [Internet]. Cham: Springer International Publishing; 2016. 166–75.
Lang N, Zhang Y, Zhang E, Zhang J, Chow D, Chang P, et al. Differentiation of spinal metastases originated from lung and other cancers using radiomics and deep learning based on DCE-MRI. Magn Reson Imaging. 2019.
Cheng C-T, Ho T-Y, Lee T-Y, Chang C-C, Chou C-C, Chen C-C, et al. Application of a deep learning algorithm for detection and visualization of hip fractures on plain pelvic radiographs. Eur Radiol [Internet]. 2019 [cited 2019 May 4]; Available from: https://doi.org/10.1007/s00330-019-06167-y.
Chung SW, Han SS, Lee JW, Oh K-S, Kim NR, Yoon JP, et al. Automated detection and classification of the proximal humerus fracture by using deep learning algorithm. Acta Orthop. 2018;89:468–73.
Belharbi S, Chatelain C, Hérault R, Adam S, Thureau S, Chastan M, et al. Spotting L3 slice in CT scans using deep convolutional network and transfer learning. Comput Biol Med. 2017;87:95–103.
MURA Dataset: Towards Radiologist-Level Abnormality Detection in Musculoskeletal Radiographs [Internet]. [cited 2019 May 4]. Available from: https://stanfordmlgroup.github.io/competitions/mura/.
Antony J, McGuinness K, Connor NEO, Moran K. Quantifying Radiographic Knee Osteoarthritis Severity using Deep Convolutional Neural Networks. ArXiv160902469 Cs [Internet]. 2016 [cited 2019 Jan 21]; Available from: http://arxiv.org/abs/1609.02469.
Tiulpin A, Thevenot J, Rahtu E, Lehenkari P, Saarakkala S. Automatic knee osteoarthritis diagnosis from plain radiographs: a deep learning-based approach. Sci Rep [Internet]. 2018 [cited 2018 Nov 19];8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5789045/.
Lu J-T, Pedemonte S, Bizzo B, Doyle S, Andriole KP, Michalski MH, et al. DeepSPINE: Automated Lumbar Vertebral Segmentation, Disc-level Designation, and Spinal Stenosis Grading Using Deep Learning. ArXiv180710215 Cs [Internet]. 2018 [cited 2018 Nov 11]; Available from: http://arxiv.org/abs/1807.10215.
Lootus M. Automated radiological analysis of spinal MRI. Ph.D. Thesis [Internet]. University of Oxford; 2015. Available from: http://www.robots.ox.ac.uk/~vgg/publications/2015/Lootus15/lootus15.pdf.
Koitka S, Demircioglu A, Kim MS, Friedrich CM, Nensa F. Ossification area localization in pediatric hand radiographs using deep neural networks for object detection. Najarian K, editor. PLOS ONE. 2018;13:e0207496.
Larson DB, Chen MC, Lungren MP, Halabi SS, Stence NV, Langlotz CP. Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology. 2018;287:313–22.
Halabi SS, Prevedello LM, Kalpathy-Cramer J, Mamonov AB, Bilbily A, Cicero M, et al. The RSNA pediatric bone age machine learning challenge. Radiology. 2018;290:498–503.
Iglovikov V, Rakhlin A, Kalinin AA, Shvets A. Pediatric bone age assessment using deep convolutional neural networks. 2018 [cited 2018 Nov 12]; Available from: http://biorxiv.org/lookup/doi/10.1101/234120.
Spampinato C, Palazzo S, Giordano D, Aldinucci M, Leonardi R. Deep learning for automated skeletal bone age assessment in X-ray images. Med Image Anal. 2017;36:41–51.
Lee H, Tajmir S, Lee J, Zissen M, Yeshiwas BA, Alkasab TK, et al. Fully automated deep learning system for bone age assessment. J Digit Imaging. 2017;30:427–41.
Tajmir SH, Lee H, Shailam R, Gale HI, Nguyen JC, Westra SJ, et al. Artificial intelligence-assisted interpretation of bone age radiographs improves accuracy and decreases variability. Skelet Radiol. 2019;48:275–83.
Kapinski N, Zielinski J, Borucki BA, Trzcinski T, Ciszkowska-Lyson B, Nowinski KS. Estimating Achilles tendon healing progress with convolutional neural networks. ArXiv180605091 Cs [Internet]. 2018 [cited 2018 Nov 11]; Available from: http://arxiv.org/abs/1806.05091.
Yune S, Lee H, Kim M, Tajmir SH, Gee MS, Do S. Beyond Human Perception: Sexual Dimorphism in Hand and Wrist Radiographs Is Discernible by a Deep Learning Model. J Digit Imaging [Internet]. 2018 [cited 2019 Apr 8]; Available from: http://link.springer.com/10.1007/s10278-018-0148-x.
Kim JR, Shim WH, Yoon HM, Hong SH, Lee JS, Cho YA, et al. Computerized bone age estimation using deep learning-based program: evaluation of the accuracy and efficiency. Am J Roentgenol. 2017;209:1374–80.
Jamaludin A, Lootus M, Kadir T, Zisserman A, Urban J, Battié MC, et al. ISSLS PRIZE IN BIOENGINEERING SCIENCE 2017: automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur Spine J. 2017;26:1374–83.
Wndchrm – an open-source utility for biological image analysis | Source Code for Biology and Medicine | Full Text [Internet]. [cited 2019 Apr 23]. Available from: https://scfbm.biomedcentral.com/articles/10.1186/1751-0473-3-13.
Winklhofer S, Held U, Burgstaller JM, Finkenstaedt T, Bolog N, Ulrich N, et al. Degenerative lumbar spinal canal stenosis: intra- and inter-reader agreement for magnetic resonance imaging parameters. Eur Spine J. 2017;26:353–61.
Miskin N, Gaviola GC, Huang RY, Kim CJ, Lee TC, Small KM, et al. Intra- and Intersubspecialty variability in lumbar spine MRI interpretation: a multireader study comparing musculoskeletal radiologists and Neuroradiologists. Curr Probl Diagn Radiol. 2019.
Machine Learning and the Future of Radiology: How we won the 2017 RSNA ML Challenge [Internet]. 16 Bit Blog. [cited 2019 Apr 20]. Available from: http://www.16bit.ai/blog/ml-and-future-of-radiology.
Deniz CM, Xiang S, Hallyburton RS, Welbeck A, Babb JS, Honig S, et al. Segmentation of the proximal femur from MR images using deep convolutional neural networks. Sci Rep. 2018;8:16485.
Ambellan F. Automated segmentation of knee bone and cartilage combining statistical shape knowledge and convolutional neural networks: data from the osteoarthritis initiative. Med Image Anal. 2019;10.
Chmelik J, Jakubicek R, Walek P, Jan J, Ourednicek P, Lambert L, et al. Deep convolutional neural network-based segmentation and classification of difficult to define metastatic spinal lesions in 3D CT data. Med Image Anal. 2018;49:76–88.
Tack A, Mukhopadhyay A, Zachow S. Knee menisci segmentation using convolutional neural networks: data from the osteoarthritis initiative. Osteoarthr Cartil. 2018;26:680–8.
Liu F, Zhou Z, Jang H, Samsonov A, Zhao G, Kijowski R. Deep convolutional neural network and 3D deformable approach for tissue segmentation in musculoskeletal magnetic resonance imaging. Magn Reson Med. 2018;79:2379–91.
Pedoia V, Majumdar S, Link TM. Segmentation of joint and musculoskeletal tissue in the study of arthritis. Magn Reson Mater Phys Biol Med. 2016;29:207–21.
Wang Y, Qiu Y, Thai T, Moore K, Liu H, Zheng B. A two-step convolutional neural network-based computer-aided detection scheme for automatically segmenting adipose tissue volume depicting on CT images. Comput Methods Prog Biomed. 2017;144:97–104.
Prasoon A, Petersen K, Igel C, Lauze F, Dam E, Nielsen M. Deep feature learning for knee cartilage segmentation using a triplanar convolutional neural network. In: Mori K, Sakuma I, Sato Y, Barillot C, Navab N, editors. Med image Comput Comput-assist Interv – MICCAI 2013. Springer: Berlin Heidelberg; 2013. p. 246–53.
Pröve P-L, Jopp-van Well E, Stanczus B, Morlock MM, Herrmann J, Groth M, et al. Automated segmentation of the knee for age assessment in 3D MR images using convolutional neural networks. Int J Legal Med 2018.
Zhou Z, Zhao G, Kijowski R, Liu F. Deep convolutional neural network for segmentation of knee joint anatomy: Zhou et al. Magn Reson Med. 2018;80:2759–70.
Badrinarayanan V, Kendall A, Cipolla R. SegNet: A deep convolutional encoder-decoder architecture for image segmentation. ArXiv151100561 Cs [Internet]. 2015 [cited 2019 Mar 24]; Available from: http://arxiv.org/abs/1511.00561
Heimann T, Morrison BJ, Styner MA, Niethammer M, Warfield SK. Segmentation of knee images: a grand challenge. Proc. MICCAI Workshop on Medical Image Analysis for the Clinic.
Trivedi H, Mesterhazy J, Laguna B, Vu T, Sohn JH. Automatic determination of the need for intravenous contrast in musculoskeletal MRI examinations using IBM Watson’s natural language processing algorithm. J Digit Imaging. 2018;31:245–51.
Jiang D, Dou W, Vosters L, Xu X, Sun Y, Tan T. Denoising of 3D magnetic resonance images with multi-channel residual learning of convolutional neural network. Jpn J Radiol. 2018;36:566–74.
Wang H, Peng H, Chang Y, Liang D. A survey of GPU-based acceleration techniques in MRI reconstructions. Quant Imaging Med Surg. 2018;8:196–208.
Chen H, Zhang Y, Zhang W, Liao P, Li K, Zhou J, et al. Low-dose CT via convolutional neural network. Biomed Opt Express. 2017;8:679–94.
Wu D, Kim K, El Fakhri G, Li Q. Iterative low-dose CT reconstruction with priors trained by artificial neural network. IEEE Trans Med Imaging. 2017;36:2479–86.
Wu Y, Ma Y, Capaldi DP, Liu J, Zhao W, Du J, et al. Incorporating prior knowledge via volumetric deep residual network to optimize the reconstruction of sparsely sampled MRI. Magn Reson Imaging. 2019.
Kang E, Min J, Ye JC. A deep convolutional neural network using directional wavelets for low-dose X-ray CT reconstruction. Med Phys. 2017;44:e360–75.
Wang S, Su Z, Ying L, Peng X, Zhu S, Liang F, et al. Accelerating magnetic resonance imaging via deep learning. 2016 IEEE 13th Int Symp Biomed Imaging ISBI. 2016. 514–7.
Hammernik K, Klatzer T, Kobler E, Recht MP, Sodickson DK, Pock T, et al. Learning a variational network for reconstruction of accelerated MRI data. ArXiv170400447 Cs [Internet]. 2017 [cited 2018 Nov 12]; Available from: http://arxiv.org/abs/1704.00447.
Galbusera F, Bassani T, Casaroli G, Gitto S, Zanchetta E, Costa F, et al. Generative models: an upcoming innovation in musculoskeletal radiology? A preliminary test in spine imaging. Eur Radiol Exp [Internet]. 2018 [cited 2018 Nov 19];2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6207611/.
Chaudhari AS, Fang Z, Kogan F, Wood J, Stevens KJ, Gibbons EK, et al. Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med. 2018;80:2139–54.
Lee YH. Efficiency improvement in a busy radiology practice: determination of musculoskeletal magnetic resonance imaging protocol using deep-learning convolutional neural networks. J Digit Imaging. 2018;31:604–10.
Trivedi H, Mesterhazy J, Laguna B, Vu T, Sohn JH. Automatic determination of the need for intravenous contrast in musculoskeletal MRI examinations using IBM Watson’s natural language processing algorithm. J Digit Imaging. 2018;31:245–51.
Chen H, Zhang Y, Kalra MK, Lin F, Chen Y, Liao P, et al. Low-dose CT with a residual encoder-decoder convolutional neural network. IEEE Trans Med Imaging. 2017;36:2524–35.
Wu Y, Ma Y, Capaldi DP, Liu J, Zhao W, Du J, et al. Incorporating prior knowledge via volumetric deep residual network to optimize the reconstruction of sparsely sampled MRI.:30.
Glockner JF, Hu HH, Stanley DW, Angelos L, King K. Parallel MR imaging: a User’s guide. RadioGraphics. 2005;25:1279–97.
Jaspan ON, Fleysher R, Lipton ML. Compressed sensing MRI: a review of the clinical literature. Br J Radiol [Internet]. 2015 [cited 2019 May 4];88. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4984938/.
He Y, Guo J, Ding X, van Ooijen PMA, Zhang Y, Chen A, et al. Convolutional neural network to predict the local recurrence of giant cell tumor of bone after curettage based on pre-surgery magnetic resonance images. Eur Radiol [Internet]. 2019 [cited 2019 Apr 8]; Available from: http://link.springer.com/10.1007/s00330-019-06082-2.
Understanding Gartner’s Hype Cycles [Internet]. [cited 2019 May 4]. Available from: https://www.gartner.com/en/documents/2538815.
Liew C. The future of radiology augmented with artificial intelligence: a strategy for success. Eur J Radiol. 2018;102:152–6.
TOUCH-AI Directory [Internet]. [cited 2019 Mar 25]. Available from: https://www.acrdsi.org/DSI-Services/TOUCH-AI.
Rubin DL, Kahn CE. Common data elements in radiology. Radiology. 2016;283:837–44.
Nguyen GK, Shetty AS. Artificial intelligence and machine learning: opportunities for radiologists in training. J Am Coll Radiol. 2018;15:1320–1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chea, P., Mandell, J.C. Current applications and future directions of deep learning in musculoskeletal radiology. Skeletal Radiol 49, 183–197 (2020). https://doi.org/10.1007/s00256-019-03284-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03284-z