Abstract
Background
Compared to other esophageal cancers, clinical stage IA esophageal cancer generally has a good prognosis, although a subgroup of patients has a poor prognosis. Unfortunately, clinical diagnoses of invasion depth or lymph node metastasis are not always accurate, which make it difficult to identify patients with a high risk of postoperative recurrence using the tumor-node-metastasis staging system. Fluorodeoxyglucose-positron emission tomography may help guide the identification of malignant tumors and the evaluation of their malignant grade based on glucose metabolism. We aimed to evaluate the association between pre-operative fluorodeoxyglucose-positron emission tomography findings and the postoperative prognosis of patients with clinical stage IA esophageal cancer.
Methods
This single-center retrospective study evaluated pre-esophagectomy fluorodeoxyglucose-positron emission tomography findings from 38 patients with clinical stage IA esophageal cancer. Receiver operating characteristic curve analysis was performed to evaluate the prognostic significance of the primary tumor having low and high SUVmax values (cut-off: 3.56).
Results
Overall survival (log-rank p = 0.034) and progression-free survival (log-rank p = 0.008) were significantly different between the groups with low SUVmax values (n = 18) and high SUVmax values (n = 20). Furthermore, the primary tumor’s SUVmax value was related to pathological vascular invasion (p = 0.045) and distant metastasis (p = 0.042).
Conclusion
The SUVmax of the primary tumor is a predictor of postoperative survival for clinical stage IA esophageal cancer. Thus, using fluorodeoxyglucose-positron emission tomography to evaluate the primary tumor’s glucose metabolism may reflect the tumor’s grade and potentially compensate for inaccuracies in tumor-node-metastasis staging.
Similar content being viewed by others
Introduction
Esophageal cancer is one of the most aggressive forms of gastrointestinal cancer and is generally treated using multimodal strategies. In Japan, neoadjuvant chemotherapy followed by surgery is recommended as curative treatment for clinical stage II–III esophageal cancer [1]. Upfront surgery remains the standard treatment for clinical stage IA (cStage IA) thoracic esophageal cancer, given its relatively good prognosis [1]. The recent emergence of diagnostic magnifying endoscopy with narrow-band imaging has also helped to identify superficial esophageal cancers [2, 3]. Thus, a remarkable proportion of cases with en bloc resection have been achieved using endoscopic submucosal dissection (ESD) [4], and endoscopic treatment in Japan is indicated for cT1a and cN0M0 superficial esophageal squamous cell carcinoma with a low risk of lymph node metastasis [1].
Unfortunately, it is difficult to accurately assess the depth of invasion for superficial esophageal cancers, which is important for determining the treatment strategy [5, 6]. Moreover, although computed tomography (CT) and positron emission tomography (PET) are used to clinically stage metastasis, these modalities are not entirely accurate, and pathological assessments have shown that 11–56% of patients with cN0 esophageal cancer develop regional lymph node metastasis after esophagectomy [7, 8]. In this context, clinical staging based on the TNM factors is a classic but powerful prognostic tool that is used to select the optimal treatment(s). However, the pre-treatment diagnosis has limited accuracy, even with the use of many diagnostic modalities, and a subgroup of patients with cStage IA esophageal cancer have a poor prognosis. Thus, it is difficult to accurately and preoperatively identify patients with cStage IA esophageal cancer who have high risks of metastasis and postoperative recurrence, and new prognostic factors would be useful to identify these patients and modify their treatment.
The use of fluorodeoxyglucose in PET (FDG-PET) can help quantify the glucose metabolism of malignant tumors [9]. In esophageal cancer, FDG-PET is reportedly useful for the pre-treatment diagnosis [10], diagnosing recurrence [11], and evaluating the effects of chemotherapy and chemoradiotherapy [12,13,14]. Moreover, FDG-PET is a useful diagnostic modality even for superficial esophageal cancers [15,16,17]. Thus, FDG-PET may be useful for diagnosing and grading esophageal cancer. The present study aimed to evaluate the association between pre-operative FDG-PET findings and the postoperative prognosis of patients with cStage IA esophageal cancer.
Patients and methods
Patients
Between May 2012 and August 2016, we identified 48 consecutive patients who underwent radical esophagectomy with two- or three-field lymphadenectomy, without neoadjuvant chemotherapy and radiotherapy, for cStage IA esophageal cancer at our department of gastroenterological surgery (Saitama Medical University International Medical Center, Saitama, Japan). The histological type was squamous cell carcinoma in all cases. The eligibility criterion was available FDG-PET findings from before the resection of local lesions in the thoracic esophagus via surgery or ESD. Therefore, we excluded 10 patients that had not undergone pre-treatment FDG-PET, and ultimately included 38 patients in the study, including 5 patients who underwent esophagectomy as an additional radical treatment after ESD. The patients’ medical records were reviewed to determine their clinicopathological characteristics and postoperative outcomes. The patients were divided according to their maximum standardized uptake value (SUVmax) of the primary tumor (SPT) into low-SPT and high-SPT groups. The study’s retrospective protocol was approved by our institutional review board (IRB number 19-002).
Clinical TNM staging and follow-up after surgery
Tumor staging was performed according to the eighth edition of the international union against cancer guidelines [18]. The pathological depth of cancer invasion was classified according to the Japanese Classification of Esophageal Cancer guidelines [19] and was comprehensively evaluated based on magnifying endoscopy with narrow-band imaging, ultrasound endoscopy, and upper gastrointestinal tract radiography. Any metastases from the neck to the abdomen were identified using CT and FDG-PET. The patients completed follow-up CT assessments of the neck, chest, and abdomen every 2–4 months after surgery for the first 3 years and then every 6 months thereafter, and esophagogastroduodenoscopy was performed annually until postoperative recurrence was suspected.
FDG-PET
The FDG-PET was performed before the resection of the primary lesion (median ± SD: 31 ± 19.5 days). Patients fasted for ≥ 6 h before the intravenous injection of FDG (3.7 MBq/kg), and approximately 60 min later the PET/CT images were acquired using Biograph 6 or Biograph 16 scanners (Siemens, Erlangen, Germany). Images from the head to the thigh were acquired for 2–3 min per bed position and reconstructed using the ordered subset expectation maximization algorithm with 3 iterations and 8 subsets. The attenuation correction was performed using transmission CT. Experienced nuclear medicine physicians drew the volume of interest on the primary tumor, and its SUVmax was measured using a workstation. If the tumor could not be identified using the PET images, the volume of interest was drawn on the specific area of the esophagus where the tumor was identified using endoscopy.
Statistical analyses
Receiver operating characteristic (ROC) curve analysis was conducted to identify the optimal SPT cut-off value for predicting postoperative recurrence. The groups were compared using the Chi-squared test or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables, as appropriate. Multiple group comparisons were performed using analysis of variance followed by Scheffe’s post-hoc test. The Kaplan–Meier method and log-rank test were used to evaluate differences in postoperative overall survival (OS) and progression-free survival (PFS), which were calculated from the date of the esophagectomy. Hazard ratios for the univariate survival analyses were calculated using a stratified Cox proportional hazards model. Differences were considered statistically significant at two-tailed p values of < 0.05. All statistical analyses were performed using SPSS software (version 24.0; IBM Corp., Armonk, NY).
Results
Patient characteristics and SUVmax
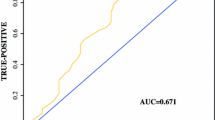
The characteristics of the 38 patients are shown in Table 1. Figure 1 shows a histogram of the SPT, which had a median value of 3.85 (range 1.3–12.3). The ROC curve analysis for predicting postoperative recurrence revealed an area under the curve of 0.58, sensitivity of 76.9%, and specificity of 60.0% (Fig. 1). The optimal cut-off value for the SPT was determined to be 3.56, which resulted in 18 patients being assigned to the low-SPT group and 20 patients being assigned to the high-SPT group.
Using the SUVmax to predict postoperative recurrence of clinical stage IA thoracic esophageal cancer in 38 patients. a A histogram of the primary tumors’ maximum standard uptake value (SUVmax), with a median value of 3.85 (range 1.3–12.3), a mean value of 4.24, and a standard deviation value of 2.28. The red dotted line shows the cut-off value (3.56) determined by receiver operating characteristic, and the black dotted line shows the mean value plus half a standard deviation value (5.38). b Receiver operating characteristic curve for predicting postoperative recurrence, with an area under the curve of 0.58, a cut-off value of 3.65, sensitivity of 76.9%, and specificity of 60.0%. The diagonal dotted line indicates the reference line
Relationships between low-SPT or high-SPT grouping and clinicopathological factors
Fischer’s test revealed no significant inter-group differences in the categorical characteristics of the low-SPT and high-SPT groups (Table 1). The Mann–Whitney U test of the continuous variables only revealed a significant inter-group difference in the presence or absence of vascular invasion (p = 0.045) (Fig. 2).
Relationship between the SUVmax and major clinical factors. The clinical factors were the presence or absence of vascular invasion (a), depth of tumor invasion (b), and presence or absence of pathological lymph node metastasis (c). The only significant inter-group difference was observed for the presence or absence of vascular invasion (Mann–Whitney U test; p = 0.045). SUVmax maximum standard uptake value of the primary tumor
Relationships between low-SPT or high-SPT grouping and postoperative survival
The mean follow-up period after surgery was 47.2 months, and 13 patients (34.2%) developed postoperative recurrence during that time. There were significant differences between the low-SPT and high-SPT groups in OS (log-rank p = 0.034) and PFS (log-rank p = 0.008) (Fig. 3). The univariate analyses also revealed that prognosis was predicted by pathological depth of tumor invasion, pathological lymph node metastasis, and vascular invasion (Table 2).
Kaplan–Meier curves for overall survival (a) and progression-free survival (b). Patients with a high SUVmax (> 3.56) had poorer prognoses than patients with a low SUVmax (< 3.56) based on the log-rank test (overall survival, p = 0.034; progression-free survival, p = 0.008). SUVmax maximum standard uptake value of the primary tumor
Relationships between low-SPT or high-SPT grouping and initial postoperative recurrence
Among the 13 patients who experienced postoperative recurrence, the initial recurrences were classified as loco-regional recurrence or systemic recurrence. We compared the SPT values between the groups with no recurrence, only loco-regional recurrence, and only systemic recurrence. There was no significant difference between the groups with no recurrence and only loco-regional recurrence, although a comparison of the groups with no recurrence and only systemic recurrence revealed that high-SPT values were significantly more common in the systemic recurrence group (p = 0.042) (Table 3).
Differences in clinicopathological findings between patients with the highest SPT and other patients
Given that the histogram did not exhibit a normal distribution, we focused on the eight people whose SPT values were higher than the mean value plus half a standard deviation value (≥ 5.38) (Table 4). Relative to the other patients, these eight patients had higher frequencies of vascular invasion, tumor submucosal invasion, lymph node metastasis, and postoperative recurrence. However, the only statistically significant difference was observed for the tumor’s infiltration into the deep submucosa (Online resource 1).
Discussion
The present study revealed that pre-operative FDG-PET findings could predict the postoperative prognosis of cStage IA thoracic esophageal cancer in the univariate analyses. Furthermore, the results suggest that high-or low-SPT values are related to pathological vascular invasion and distant metastasis. Although some studies have already examined the diagnostic utility of PET, many of those studies had examined advanced and recurrent esophageal cancers [20,21,22,23]. In addition, most studies of superficial or cStage IA esophageal cancers have examined the relevance of pathological factors in the resected specimen, such as tumor invasion depth or lymph node metastasis [15,16,17]. Moreover, only a few studies have examined factors predicting the postoperative prognosis of esophageal cancer, and those studies examined relatively small samples from single centers [24]. Thus, the association between PET findings and long-term prognosis for cStage IA esophageal cancer remains unclear. Our study revealed that the SPT values may be related to vascular invasion status, but not to any other clinicopathological factor. It is possible that vascular invasion contributes to the risk of distant organ metastasis, which has a poor prognosis. The fact that high-SPT values were also related to the incidence of systemic recurrence (as the first type of recurrence) also appears to support this biological hypothesis of pathological vascular invasion. These findings indicate that SPT values, rather than the most established clinicopathological factors (e.g., pathological depth of tumor invasion or lymph node metastasis), may be more useful for predicting the postoperative recurrence of esophageal cancer based on glucose metabolism, similar to vascular invasion. Nevertheless, previous studies have indicated that lymphatic invasion is a good predictor of lymph node metastasis in superficial esophageal cancers [25, 26]. Although lymphatic invasion is usually more common than lymph node metastasis, we observed that lymphatic invasion was less common than lymph node metastasis. However, as shown in online resource 2, it is necessary to consider the sub-classification of cancer invasion depth and histology, as there is broad inter-center variation in the pathological diagnoses for superficial esophageal cancer [16, 24, 26,27,28,29,30,31,32,33]. The relatively low sensitivity for the presence of lymphatic invasion at our institution may have affected the association between lymphatic invasion and SPT.
A comparison of the eight patients with the highest SPT values to the other patients revealed that the only significant relationship was with tumor depth invasion (online resource 1). In this context, FDG-PET is thought to reflect the tumor’s volume and activity [34], and Nakajima et al. reported that, using a SPT cut-off of 2.5 for superficial esophageal cancer, 82% of high-SPT tumors invaded deep into the submucosa [17]. Given that SPT depends on both malignancy potential and tumor volume (reflected in the tumor’s depth of invasion), the eight patients with the highest SPT values seemed to have a greater tumor burden. This may be related to rates of lymph node metastasis and postoperative recurrence that are similar to those of normal clinical T2N0 cases. A preliminary review of 57 clinical Stage II/III patients who underwent esophagectomy after neoadjuvant chemotherapy at our institution during the same period revealed that these patients had a much higher SPT (mean ± SD 11.3 ± 5.16). This suggests that the higher cut-off values, such as for the top 8 patient group, are verystrong for predicting tumor depth invasion, and that the optimal cut-off value should be lower for predicting prognosis in cases of cStage IA esophageal cancer. Nevertheless, the fact that our cut-off value of 3.56 did not correlate with tumor depth may support the appropriateness of our cut-off value for cStage IA patients. It would of course be necessary to identify and confirm the appropriate cut-off value for patients with advanced cancer.
An inaccurate pre-treatment diagnosis of lymph node metastasis can confound the selection of a treatment strategy for cStage IA esophageal cancer. For example, a pathological examination revealed that lymph node metastasis was present in approximately 27.0% of resected specimens from cases with cStage IA thoracic esophageal cancer [35]. Moreover, the frequency of lymph node metastasis increases proportionally to the depth of invasion [36], and it is important to accurately determine the depth of invasion before selecting the treatment strategy for cN0 cases. However, the depth of invasion is difficult to accurately determine for superficial esophageal cancer [5, 6], and diagnostic ESD has been considered for cT1 esophageal cancers [37]. Therefore, it would be preferable to have novel modalities or biomarkers that can complement the clinical TNM factors for cStage IA esophageal cancer, as this would presumably allow for more accurate staging of esophageal cancers. Our findings indicate that the significant prognostic factors were the presence or absence of pathological lymph node metastasis, vascular invasion and the pre-operative SPT value in univariate analysis. However, pathological lymph node metastasis and vascular invasion are postoperative diagnostic factors, and the SPT value is more useful for pre-operative prognostication, which may make it ideal for supplementing the selection of a treatment strategy based on the clinical TNM factors. For example, cStage IA patients with a high risk of recurrence based on the pre-operative PET findings may be suitable for pre-operative therapy that is currently used in cStage II/III cases. Our results revealed that the high-SPT group had a similar prognosis to Japanese cases with pathological stage II/III, which suggests that FDG-PET can be used to identify cStage IA cases with a relatively high risk of recurrence. In Japan, neoadjuvant chemotherapy has already been shown to be superior to adjuvant chemotherapy for clinical stage II/III esophageal cancer [38], which suggests that the high-SPT group of cStage IA cases might benefit from neoadjuvant chemotherapy. However, one study also revealed that FDG-PET might not predict a survival benefit in patients with locally advanced inoperable esophageal cancer [39]. It is possible that identifying cStage IA patients with a high risk of recurrence based on the pre-operative FDG-PET findings may allow physicians to proactively introduce multidisciplinary treatment and improve their prognosis.
The present study is clearly limited by its single-center retrospective design, small sample size, and relatively short follow-up period. In particular, the small sample size and the number of events precluded a multivariate analysis using the Cox hazard model. Therefore, only the univariate analyses revealed that SPT was related to postoperative survival. Thus, larger prospective studies are needed to validate our findings. Furthermore, the sample of 38 patients may be insufficient to determine the cut-off value based on the ROC, as the SPT values were not normally distributed and the ROC curve’s area was not very large (Fig. 1), which may have influenced our selection of the “optimal” SPT cut-off value. Moreover, similar PET-based studies use a SUVmax value of approximately 2.5, which reflects the mediastinum background. However, the median SUVmax value in our study was 3.85, and using the same cut-off values from the previous studies would have assigned more than half of cases to the high-SPT group (which is expected to be a small group and is assumed to have a poor prognosis). Thus, we believe that this approach would not have been appropriate, and we used the ROC to set a non-arbitrary cut-off value that was not based on the mediastinum background. Therefore, larger studies are also needed to identify the best cut-off value for identifying potential malignancy and complementing the TNM factors. Other studies have also evaluated different PET parameters, such as the metabolic tumor volume change [40], which may be more practical than using the SUVmax.
In conclusion, the findings of this study indicate that the SPT value from pre-operative PET could predict postoperative survival for cStage IA esophageal cancer. It is possible that glucose metabolism, evaluated using PET, may reflect the tumor’s grade and potentially compensate for inaccuracies in the clinical TNM staging of cStage IA esophageal cancer.
References
Kitagawa Y, Uno T, Oyama T et al (2019) Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal society: part 1. Esophagus 16:1–24
Endo M, Kawano T (1997) Detection and classification of early squamous cell esophageal cancer. Dis Esophagus 10:155–158
Muto M, Minashi K, Yano T et al (2010) Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 28:1566–1572
Ishihara R, Iishi H, Uedo N et al (2008) Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 68:1066–1072
Katada C, Tanabe S, Wada T et al (2019) Retrospective assessment of the diagnostic accuracy of the depth of invasion by narrow band imaging magnifying endoscopy in patients with superficial esophageal squamous cell carcinoma. J Gastrointest Cancer 50:292–297
Mizumoto T, Hiyama T, Oka S et al (2018) Diagnosis of superficial esophageal squamous cell carcinoma invasion depth before endoscopic submucosal dissection. Dis Esophagus. https://doi.org/10.1093/dote/dox142
McGuill MJ, Byrne P, Ravi N et al (2008) The prognostic impact of occult lymph node metastasis in cancer of the esophagus or esophago-gastric junction: systematic review and meta-analysis. Dis Esophagus 21:236–240
Aoyama J, Kawakubo H, Mayanagi S et al (2019) Discrepancy between the clinical and final pathological findings of lymph node metastasis in superficial esophageal cancer. Ann Surg Oncol 26:2874–2881
Rigo P, Paulus P, Kaschten BJ et al (1996) Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur J Nucl Med 23:1641–1674
Kato H, Kuwano H, Nakajima M et al (2002) Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 94:921–928
Guo H, Zhu H, Xi Y et al (2007) Diagnostic and prognostic value of 18F-FDG PET/CT for patients with suspected recurrence from squamous cell carcinoma of the esophagus. J Nucl Med 48:1251–1258
Brücher BL, Weber W, Bauer M et al (2001) Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg 233:300–309
Downey RJ, Akhrust T, Ilson D et al (2003) Whole body 18FDGPET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol 21:428–432
Miyata H, Yamasaki M, Takahashi T et al (2014) Determinants of response to neoadjuvant chemotherapy for esophageal cancer using 18F-fluorodeoxiglucose positron emission tomography (18F-FDG-PET). Ann Surg Oncol 21:575–582
Miyata H, Doki Y, Yasuda T et al (2008) Evaluation of clinical significance of 18F-fluorodeoxyglucose positron emission tomography in superficial squamous cell carcinomas of the thoracic esophagus. Dis Esophagus 21:144–150
Kita Y, Okumura H, Uchikado Y et al (2013) Clinical significance of 18F-fluorodeoxyglucose positron emission tomography in superficial esophageal squamous cell carcinoma. Ann Surg Oncol 20:1646–1652
Nakajima M, Muroi H, Yokoyama H et al (2018) 18 F-Fluorodeoxyglucose positron emission tomography can be used to determine the indication for endoscopic resection of superficial esophageal cancer. Cancer Med 7:3604–3610
Rice TW, Gress DM, Patil DT et al (2017) Cancer of the esophagus and esophagogastric junction-major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:304–317
Japan Esophageal Society (2017) Japanese Classification of Esophageal Cancer, 11th edition: part I. Esophagus 14:1–36
Brown C, Howes B, Jamieson GG et al (2012) Accuracy of PET-CT in predicting survival in patients with esophageal cancer. World J Surg 36:1089–1095
Yasuda T, Yano M, Miyata H et al (2015) Prognostic significance of (18)F-fluorodeoxyglucose positron emission tomography (FDG-PET)-positive lymph nodes following neoadjuvant chemotherapy and surgery for resectable thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 22:2599–2607
Hamai Y, Hihara J, Emi M et al (2016) Ability of fluorine-18 fluorodeoxyglucose positron emission tomography to predict outcomes of neoadjuvant chemoradiotherapy followed by surgical treatment for esophageal squamous cell carcinoma. Ann Thorac Surg 102:1132–1139
Izumi D, Yoshida N, Watanabe M et al (2016) Tumor/normal esophagus ratio in (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for response and prognosis stratification after neoadjuvant chemotherapy for esophageal squamous cell carcinoma. J Gastroenterol 51:788–795
Furukawa T, Hamai Y, Hihara J et al (2016) Clinical significance of FDG-PET to predict pathologic tumor invasion and lymph node metastasis of superficial esophageal squamous cell carcinoma. Ann Surg Oncol 23:4086–4092
Nakajima Y, Nagai K, Miyake S et al (2002) Evaluation of an indicator for lymph node metastasis of esophageal squamous cell carcinoma invading the submucosal layer. Jpn J Cancer Res. 93(3):305–312
Shimada H, Nabeya Y, Matsubara H et al (2006) Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 191(2):250–254
Stein HJ, Feith M, Bruecher BL et al (20015) Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 242(4):566–573 (discussion 573–5)
Altorki NK, Lee PC, Liss Y et al (2008) Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg. 247(3):434–439. https://doi.org/10.1097/SLA.0b013e318163a2ff
Ancona E, Rampado S, Cassaro M et al (2008) Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 15(11):3278–3288. https://doi.org/10.1245/s10434-008-0065-1(Epub 2008 Aug 26)
Tomita N, Matsumoto T, Hayashi T et al (2008) Lymphatic invasion according to D2–40 immunostaining is a strong predictor of nodal metastasis in superficial squamous cell carcinoma of the esophagus: algorithm for risk of nodal metastasis based on lymphatic invasion. Pathol Int. 58(5):282–287. https://doi.org/10.1111/j.1440-1827.2008.02224.x
Tachibana M, Hirahara N, Kinugasa S et al (2008) Clinicopathologic features of superficial esophageal cancer: results of consecutive 100 patients. Ann Surg Oncol. 15(1):104–116 (Epub 2007 Sep 22)
Chiba T, Kawachi H, Kawano T et al (2010) Independent histological risk factors for lymph node metastasis of superficial esophageal squamous cell carcinoma; implication of claudin-5 immunohistochemistry for expanding the indications of endoscopic resection. Dis Esophagus. 23(5):398–407. https://doi.org/10.1111/j.1442-2050.2009.01023.x(Epub 2009 Nov 9)
Zhuge L, Wang S, Xie J et al (2018) A model based on endoscopic morphology of submucosal esophageal squamous cell carcinoma for determining risk of metastasis on lymph nodes. J Thorac Dis. 10(12):6846–6853. https://doi.org/10.21037/jtd.2018.11.77
Kato H, Miyazaki T, Nakajima M et al (2005) The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer 103(1):148–156
Akutsu Y, Kato K, Igaki H et al (2016) The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg 264:1009–1015
Yamashina T, Ishihara R, Nagai K et al (2013) Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am J Gastroenterol 108:544–551
Kurokawa Y, Muto M, Minashi K et al (2009) A phase II trial of combined treatment of endoscopic mucosal resection and chemoradiotherapy for clinical stage I esophageal carcinoma: Japan Clinical Oncology Group Study JCOG0508. Jpn J Clin Oncol 39:686–689
Ando N, Kato H, Igaki H et al (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 19(1):68–74. https://doi.org/10.1245/s10434-011-2049-9(Epub 2011 Aug 31)
Jingu K, Umezawa R, Yamamoto T et al (2019) FDG-PET might not contribute to improving survival in patients with locally advanced inoperable esophageal cancer. Int J Clin Oncol 24:927–933
Makino T, Yamasaki M, Tanaka K et al (2019) (2018) Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg 270(6):1090–1095. https://doi.org/10.1097/SLA.0000000000002808
Acknowledgements
The authors thank Dr. Yukihiro Watanabe (a biostatistician) for advice on statistical methods.
Author information
Authors and Affiliations
Contributions
YM: Study conception and design, collecting, analyzing, and interpreting the data, and writing the manuscript. HS: Study conception and design. NF: Collecting the data. SO: Collecting the data. HS: Collecting the data. YH: Collecting the data. TY: Analyzing and interpreting the data. SS: Critical intellectual contributions. KO: Critical intellectual contributions. SY: Critical intellectual contributions. IK: Study supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Formal consent was not required for this type of study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Miyawaki, Y., Sato, H., Fujiwara, N. et al. Association of the primary tumor’s SUVmax with survival after surgery for clinical stage IA esophageal cancer: a single-center retrospective study. Int J Clin Oncol 25, 561–569 (2020). https://doi.org/10.1007/s10147-019-01606-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01606-8