Abstract
Introduction
We retrospectively evaluated the blood coagulation activity using the D-dimer level in the early period after gastrectomy and investigated whether postoperative hypercoagulation affects tumor recurrence and long-term survival in gastric cancer patients.
Methods
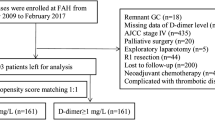
The study involved 650 patients who underwent curative resection for gastric cancer at Kanagawa Cancer Center between July 2009 and July 2013. They were divided into a low-D-dimer group (LD group) and high-D-dimer group (HD group) according to the median D-dimer level on postoperative day (POD) 7. The risk factors for overall survival (OS) and relapse-free survival (RFS) were identified.
Results
Of the 448 enrolled patients, 218 were classified into the LD group and 230 into the HD group. The 5-year OS rates after surgery were 90.8% and 81.3% in the LD and HD groups, respectively (p < 0.001). The 5-year RFS rates after surgery were 89.9% and 76.1% in the LD and HD groups, respectively (p < 0.001). A high D-dimer level on POD 7 (≥ 4.9 μg/ml) was identified as an independent predictive factor for both the OS (hazard ratio [HR] 1.955, 95% confidence interval [CI] 1.158–3.303, p = 0.012) and RFS (HR 2.182, 95% CI 1.327–3.589, p = 0.002). Furthermore, hematological recurrence was significantly more frequent in the HD group than in the LD group (p = 0.014).
Conclusion
A high D-dimer level on POD 7 may predict tumor recurrence and the long-term survival in patients who undergo gastrectomy for locally advanced gastric cancer. Patients with an elevated postoperative D-dimer level need careful observation and diagnostic imaging to timely detect tumor recurrence.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–386
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Tsuburaya A, Mizusawa J, Tanaka Y et al (2014) Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 101:653–660
Al-Batran SE, Homann N, Pauligk C et al (2019) Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. https://doi.org/10.1016/s0140-6736(18)32557-1
Ay C, Dunkler D, Pirker R et al (2012) High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica 97:1158–1164
Wojtukiewicz MZ, Hempel D, Sierko E et al (2016) Thrombin-unique coagulation system protein with multifaceted impacts on cancer and metastasis. Cancer Metastasis Rev 35:213–233
Palumbo JS, Talmage KE, Massari JV et al (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105:178–185
Gay LJ, Felding-Habermann B (2011) Contribution of platelets to tumour metastasis. Nat Rev Cancer 11:123–134
Fukumoto K, Taniguchi T, Usami N et al (2015) Preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today 45:63–67
Kilic L, Yildiz I, Sen FK et al (2015) D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark 15:405–411
Liu P, Zhu Y, Liu L (2015) Elevated pretreatment plasma D-dimer levels and platelet counts predict poor prognosis in pancreatic adenocarcinoma. Onco Targets Ther 8:1335–1340
Xu L, He F, Wang H et al (2017) A high plasma D-dimer level predicts poor prognosis in gynecological tumors in East Asia area: a systematic review and meta-analysis. Oncotarget 8:51551–51558
Yu J, Li D, Lei D et al (2016) Tumor-specific D-dimer concentration ranges and influencing factors: a cross-sectional study. PLoS ONE 11:e0165390
Diao D, Cheng Y, Song Y et al (2017) D-dimer is an essential accompaniment of circulating tumor cells in gastric cancer. BMC Cancer 17:56
Ay C, Vormittag R, Dunkler D et al (2009) D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 27:4124–4129
Diao D, Wang Z, Cheng Y et al (2014) D-dimer: not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS ONE 9:e101125
Liu L, Zhang X, Yan B et al (2014) Elevated plasma D-dimer levels correlate with long term survival of gastric cancer patients. PLoS ONE 9:e90547
Kanda M, Tanaka C, Kobayashi D et al (2017) Proposal of the coagulation score as a predictor for short-term and long-term outcomes of patients with resectable gastric cancer. Ann Surg Oncol 24:502–509
Association JGC (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Japanese Gastric Cancer A (2017) Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 20:1–19
Yamada T, Hayashi T, Cho H et al (2012) Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer 15:34–41
Dindo D, Demartines N, Clavien P-A (2004) Classification of Surgical Complications. Ann Surg 240:205–213
Tanizawa Y, Bando E, Kawamura T et al (2017) Prevalence of deep venous thrombosis detected by ultrasonography before surgery in patients with gastric cancer: a retrospective study of 1140 consecutive patients. Gastric Cancer 20:878–886
Kodama J, Seki N, Fukushima C et al (2013) Postoperative decreased levels of D-dimer in patients with gynecologic cancer with enoxaparin and fondaparinux thromboprophylaxis. Mol Clin Oncol 1:737–744
Tsuji K, Eguchi Y, Kodama M (1996) Postoperative hypercoagulable state followed by hyperfibrinolysis related to wound healing after hepatic resection. J Am Coll Surg 183:230–238
Wang Z, Fu J, Diao D et al (2011) Plasma D-dimer level in the perioperative period in non-small-cell lung cancer. Thorac Cancer 2:207–212
Acknowledgements
This work was supported, in part, by the non-governmental organization Yokohama Surgical Research Group, Dr. Masumi Kamachi (Tokyo Shinagawa Hospital), Dr. Ryuji Tominaga (Fukuoka Wajiro Hospital), Dr. Nobuko Yoshiki (Yoshiki Dermatology Clinic Ginza). The authors express their sincere gratitude to Ms. Natsumi Sato and Ms. Rika Takahashi for their excellent data management in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hara, K., Aoyama, T., Hayashi, T. et al. Postoperative D-dimer elevation affects tumor recurrence and the long-term survival in gastric cancer patients who undergo gastrectomy. Int J Clin Oncol 25, 584–594 (2020). https://doi.org/10.1007/s10147-019-01603-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01603-x