Abstract
Background
Fluoropyrimidine plus platinum, followed by paclitaxel (PTX) plus ramucirumab is a recommended treatment strategy for advanced gastric cancer (AGC). We investigated how peripheral neuropathy (PN), induced by platinum in first-line chemotherapy, affected the tolerability of second-line chemotherapy with PTX (2nd-PTX).
Methods
The subjects were AGC patients who received second-line chemotherapy with PTX (2nd-PTX) after the failure of platinum-based chemotherapy between March 2015 and June 2018. We retrospectively reviewed PN severity, and dose reduction and/or discontinuation due to PN during 2nd-PTX, and compared the cumulative incidence of grade 2 PN between the two groups according to first-line chemotherapy containing oxaliplatin (L-OHP) or cisplatin (CDDP).
Results
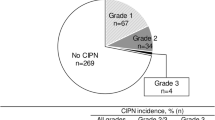
The L-OHP and CDDP groups consisted of 50 patients each. PN severity before 2nd-PTX was grade 1/2 in 46/12% of patients in the L-OHP group, and 100/0% in the CDDP group. The worst grades of chemotherapy-induced PN during 2nd-PTX were grades 1/2/3 in 40/34/14% of patients in the L-OHP group, and 36/18/0% in the CDDP group. Median time to grade 2 PN after starting second-PTX was 2.5 months in the L-OHP group and 8.6 months in the CDDP group (hazard ratio 3.34, p = 0.002). The frequencies of a PN-related dose reduction and/or discontinuation of PTX were 18% in the L-OHP group and 8% in the CDDP group (p = 0.234).
Conclusions
The severity of PN and tolerability of 2nd-PTX may be affected by first-line chemotherapy with L-OHP or CDDP for AGC.




Similar content being viewed by others
References
Japanese Gastric Cancer Association (2018) Japanese gastric cancer treatment guidelines (ver. 5), Tokyo, Japan. Japanese Gastric Cancer Association, Tokyo
Blumenthal DT (2009) Assessment of neuropathy pain in cancer patients. Curr Pain Headache Rep 13(4):282–287
Hausheer FH, Schilsky RL, Bain S et al (2006) Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol 33(1):15–49
Paice JA (2009) Clinical challenges: chemotherapy-induced peripheral neuropathy. Semin Oncol Nurs. https://doi.org/10.1016/j.soncn.2009.03.013
Argyriou AA, Polychronopoulos P, Iconomou G et al (2007) Incidence and characteristics of peripheral neuropathy during oxaliplatin based chemotherapy for metastatic colon cancer. Acta Oncol 46(8):1131–1137
Hilkens PH, ven den Bent MJ (1997) Chemotherapy-induced peripheral neuropathy. J Peripher Nerv Syst 2(4):350–361
Thompson SW, Davis LE, Kornfeld M (1984) Cisplatin neuropathy. Clinical, electrophysiologic, morphologic, and toxicologic studies. Cancer 54(7):1269–1275
Gregg RW, Molepo JM, Monpetit VJ et al (1992) Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. J Clin Oncol 10(5):795–803
Glendenning JL, Barbachano Y, Norman AR et al (2010) Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 116(10):2322–2331
van der Hoop RG, van der Burg ME, ten Bokkel Huinink WW et al (1990) Incidence of neuropathy in 395 patients with ovarian cancer treated with or without cisplatin. Cancer 66(8):1697–1702
Cavaletti G, Marzorati L, Bogliun G et al (1992) Cisplatin-induced peripheral neurotoxicity is dependent on total-dose intensity and single-dose intensity. Cancer 69(1):203–207
Mollman JE, Glover DJ, Hogan WM et al (1988) Cisplatin neuropathy. Risk factors, prognosis, and protection by WR-2721. Cancer 61(11):2192–2195
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Souglakos J, Mavroudis D, Kakolyris S et al (2002) Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: a multicenter phase II trial. J Clin Oncol 20(11):2651–2657
Yamada Y, Higuchi K, Nishikawa K et al (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 26(1):141–148
Cunningham D, Starling N, Rao S et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358(1):36–46
Al-Batran SE, Hartmann JT, Probst S et al (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 26(9):1435–1442
Park SB, Goldstein D, Krishnan AV et al (2013) Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA cancer J Clin 63(6):419–437
Kang JH, Lee SI, Lim DH et al (2012) Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 30(13):1513–1518
Hironaka S, Ueda S, Yasui H et al (2013) Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 31(35):4438–4444
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48(3):452–458
Pachman DR, Qin R, Seisler DK et al (2015) Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol 33(30):3416–3422
Yamaguchi K, Kusaba H, Makiyama A et al (2018) The risk factors for oxaliplatin-induced peripheral sensory neuropathy and thrombocytopenia in advanced gastric cancer. Cancer Chemother Pharmacol 82(4):625–633
Quasthoff S, Hartung HP (2002) Chemotherapy-induced peripheral neuropathy. J Neurol 249(1):9–17
Oki E, Emi Y, Kojima H et al (2015) Preventive effect of Goshajinkigan on peripheral neurotoxicity of FOLFOX therapy (GENIUS trial): a placebo-controlled, double-blind, randomized phase III study. Int J Clin Oncol 20(4):767–775
Loprinzi CL, Qin R, Dakhil SR et al (2014) Phase III randomized, placebo-controlled, double-blind study of intravenous calcium and magnesium to prevent oxaliplatin-induced sensory neurotoxicity (N08CB/Alliance). J Clin Oncol 32(10):997–1005
Miltenburg NC, Boogerd W (2014) Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev 40(7):872–882
Basch E, Jia X, Heller G et al (2009) Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst 101(23):1624–1632
Iizumi S, Takashima A, Sakamaki K et al (2018) Survival impact of post-progression chemotherapy in advanced gastric cancer: systematic review and meta-analysis. Cancer Chemother Pharmacol 81(6):981–989
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No author has any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Otsuka, R., Iwasa, S., Yanai, T. et al. Impact of peripheral neuropathy induced by platinum in first-line chemotherapy on second-line chemotherapy with paclitaxel for advanced gastric cancer. Int J Clin Oncol 25, 595–601 (2020). https://doi.org/10.1007/s10147-019-01598-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-019-01598-5