Abstract
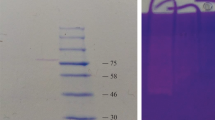
Pepsin from stomach of Bagre panamensis was semi-purified and biochemically characterized. The acid proteolytic activity and purification fold were 3875 U/mg protein and 91.85, respectively, after purification process. The optimum pH and temperature for semi-purified protease were 2–3 and 65 °C, respectively. The enzyme activity was stable after heating proteases at 50 °C for 120 min, but only 30% residual activity was detected after heating at 65 °C for 30 min. SDS-PAGE analysis showed two proteins bands after dialysis (26.1 and 38.6 kDa). Only the band of 38.6 kDa had proteolytic activity, which was inhibited using pepstatin A. Organic solvents, surfactants and reducing agents affect the proteolytic activity at different extent; however, metal ions or EDTA have no impact on protease activity. The semi-purified protease exhibited milk coagulant activity, with a maximum activity at 45 °C. The obtained results highlight the potential biotechnological use of B. panamensis pepsin.





Similar content being viewed by others
References
Anson ML. The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J. Gen. Physiol. 22:79–89 (1938)
Arvanitoyannis IS, Kassaveti A. Fish industry waste: treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 43:726–745 (2008)
Barberis S, Quiroga E, Morcelle S, Priolo N, Luco JM. Study of phytoproteases stability in aqueous-organic biphasic systems using linear free energy relationships. J. Mol. Catal. B Enzym. 38:95–103 (2006)
Bkhairia I, Mhamdi S, Jridi M, Nasri M. New acidic proteases from Liza aurata viscera: Characterization and application in gelatin production. Int. J. Biol. Macromol. 92:533–542 (2016)
Bougatef A, Balti B, Zaied SB, Soussi N, Nasri M. Pepsinogen and pepsin from the stomach of smooth hound (Mustelus mustelus): Purification, characterization and amino acid terminal sequences. Food Chem. 107:777–784 (2008)
Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of dye binding. Anal. Biochem. 72:248–254 (1976)
Burgess RR. Protein precipitation techniques. Chapter 20, pp. 331–342. In: Methods in Enzymology. Volume 463. Guide to protein purification. Burgess RR, Deutscher MP (eds). Academic Press, San Diego, CA, USA (2009)
Gildberg A. Aspartic proteinases in fishes and aquatic invertebrates. Comp. Biochem. Physiol. B 91:425–435(1988)
Harpaz S, Eshel A, Lindner P. Effect of 1-propanol on the activity of intestinal proteolytic enzymes of the European sea bass Dicentrarchus labrax. J. Agric. Food Chem. 42:49–52 (1994)
Homaei A, Lavajoo F, Sariri R. Development of marine biotechnology as a resource for novel proteases and their role in modern biotechnology. Int. J. Biol. Macromol. 88:542–552 (2016)
Jacob M, Jaros D, Rohm H. Recent advances in milk clotting enzymes. Int. J. Dairy Technol. 64:14–33 (2011)
Klomklao S, Kishimura H, Yabe M, Benjakul S. Purification and characterization of two pepsins from the stomach of pectoral rattail (Coryphaenoides pectoralis). Comp. Biochem. Physiol. B 147:682–689 (2007)
Kumar D, Bhalla TC. Microbial proteases in peptide synthesis: approaches and applications. Appl. Microbiol. Biotechnol. 68:726–736 (2005)
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 (1970)
Mazorra-Manzano MA, Moreno-Hernández JM, Ramírez Suárez JC, Torres-Llanez MJ, González-Córdova AF, Vallejo-Córdoba B. Sour orange Citrus aurantium L. flowers: a new vegetable source of milk-clotting proteases. LWT Food Sci. Technol. 54:325–330 (2013)
Miura Y, Kageyama T, Moriyama A. Pepsinogens and pepsins from largemouth bass, Micropterus salmoides: Purifications and characterization with special reference to high proteolytic activities of bass enzymes. Comp. Biochem. Physiol. B 183:42–48 (2015)
Nalinanon S, Benjakul S, Kishimura H, Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 124:1354–1362 (2011)
Nalinanon S, Benjakul S, Kishimura H. Biochemical properties of pepsinogen and pepsin from the stomach of albacore tuna (Thunnus alalunga). Food Chem. 121:49–55 (2010)
Nalinanon S, Benjakul S, Visessanguan W, Kishimura, H. Use of pepsin for collagen extraction from the skin of bigeye snapper (Priacanthus tayenus). Food Chem. 104:593–601 (2007)
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H. Improvement of gelatin extraction from bigeye snapper skin using pepsin-aided process in combination with protease inhibitor. Food Hydrocoll. 22:615–622 (2008a)
Nalinanon S, Benjakul S, Visessanguan W, Kishimura H. Tuna pepsin: Characteristics and its use for collagen extraction from the skin of threadfin bream (Nemipterus spp.). J. Food Sci. 73:C413–C419 (2008b)
Nasri RN, Younes I, Lassoued I, Ghorbel S, Ghorbel-Bellaaj O, Nasri M. Digestive alkaline proteases from Zosterisessor ophiocephalus, Raja clavata, and Scorpaena scrofa: Characteristics and application in chitin extraction. J. Amino Acids 2011:913616 (2011)
Noda M, Murakami K. Studies on proteinases from the digestive organs of sardine. II. Purification and characterization of two acid proteinases from the stomach. Biochim. Biophys. Acta 658:27–34 (1981)
Olsen RL, Toppe J, Karunasagar I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 36:144–151 (2014)
Ogino H, Ishikawa H. Enzymes which are stable in the presence of organic solvents. J. Biosci. Bioeng. 91(2):109–116 (2001)
Pereira NA, Fernández-Gimenez AV. Exogenous enzymes in dairy technology: Acidic proteases from processing discards of shrimp Pleoticus muelleri and their use as milk-clotting enzymes for cheese manufacture. Int. J. Food Sci. Technol. 52:341–347 (2017)
Saborowski R, Sahling G, Navarrete del Toro MA, Walter I, García-Carreño FL. Stability and effects of organic solvents on endopeptidases from the gastric fluid of the marine crab Cancer pagurus. J. Mol. Catal. B Enzym. 30:109–118 (2004)
Sathivel S, Yin H, Prinyawiwatkul W, King JM. Comparisons of chemical and physical properties of catfish oils prepared from different extracting processes. J. Food Sci. 74(2):E70–E76 (2009)
Shankar S, Laxman RS. Biophysicochemical characterization of an alkaline protease from Beauveria sp. MTCC 5184 with multiple applications. Appl. Biochem. Biotechnol. 175:589-602 (2015)
Simmons M, Ru G, Casalone C, Iulini B, Cassar C, Seuberlich T. Discontools: identifying gaps in controlling bovine spongiform encephalopathy. Transbound. Emerg. Dis. 65:9–21 (2018)
Stauffer C. Enzyme assays for food scientist. New York: Van Nostrand (1989)
Squires EJ, Haard F, Feltham LAW. Gastric proteases of the Greenland cod Gadus ogac. II. Structural properties. Biochem. Cell Biol. 64:215–222 (1986)
Tavares JFP, Baptista JAB, Marcone MF. Milk-coagulating enzymes of tuna fish waste as a rennet substitute. Int. J. Food Sci. Nutr. 48:169–176 (1997)
Wu T, Sun LC, Du CH, Cai QF, Zhang QB, Su WJ, Cao MJ. Identification of pepsinogens and pepsins from the stomach of European eel (Anguilla Anguilla). Food Chem. 115:137–142 (2009)
Zhao L, Budge SM, Ghaly AE, Brooks MS, Dave D. Extraction, purification and characterization of fish pepsins: a critical review. J. Food Process. Technol. 2:1–14 (2011)
Acknowledgements
The authors would like to thank to MC Gissel Rios and Reyna Tiznado for her laboratory assistance and MC Jesús Martín Moreno Hernandez for his advices for milk-clotting assays. Also, the authors thank María Elena Sánchez Salazar for her editorial work in English. This work has been elaborated as an activity from thematic research network of CONACYT: “RED 12.4, Para reducir y valorizar las pérdidas y desperdicios de alimentos: Hacia sistemas alimentarios sostenibles (No. 294768)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights statement
This article does not contain studies with animals subjects performed by the any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osuna-Ruiz, I., Espinoza-Marroquin, M.F., Salazar-Leyva, J.A. et al. Biochemical characterization of a semi-purified aspartic protease from sea catfish Bagre panamensis with milk-clotting activity. Food Sci Biotechnol 28, 1785–1793 (2019). https://doi.org/10.1007/s10068-019-00614-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00614-8