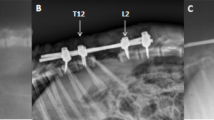
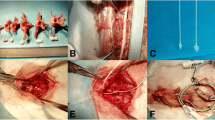
Abstract
Purpose
To determine the safe range of shortening the spinal column at middle thoracic spine and to observe the changes in blood-spinal cord barrier (BSCB), microglia/macrophage activation and inducible nitric oxide synthase (iNOS) activity after shortening-induced spinal cord injury.
Methods
Dogs were allocated to four groups. Group A (control) underwent laminectomy of T7 without shortening the spinal column. Groups B, C and D had 1/3, 1/2, and 2/3 of T7 resected, respectively, followed by spinal shortening. Somatosensory evoked potential (SSEP) and hind-limb function were recorded periodically for 14 days after operation. Spinal cord blood flow (SCBF) and BSCB were detected at the acute phase of shortening. Microglia/macrophage reactions and iNOS activity were observed by immunohistochemistry.
Results
Shortening of 1/3 of a vertebral height caused no significant changes in SSEP and hind-limb function after operation, whereas shortening of 1/2 of the height caused SSEP abnormality and paraparesis, and severe neurologic deficit of hind-limb was observed when the shortening reached 2/3 of the height. SCBF increased temporarily and showed a trend of recovery when the shortening was within 1/2 of a vertebral segment height. When it reached 1/2 or 2/3 of the height, SCBF at 6 h post-operation was 86.33% or 74.95% of the baseline, and an increasing BSCB permeability was observed. In the subsequent 7 days, obvious activation of macrophage and increased number of iNOS-positive cells were observed.
Conclusion
It is safe to shorten the spinal cord within 1/3 of a vertebral height in middle thoracic spine under two-segment laminectomy in canine. The BSCB disruption, macrophage activation, and increased iNOS activity were observed in the acute phase of the injury.
Graphic abstract
These slides can be retrieved under Electronic Supplementary Material.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Yang C, Zheng Z, Liu H, Wang J, Kim YJ, Cho S (2016) Posterior vertebral column resection in spinal deformity: a systematic review. Eur Spine J 25(8):2368–2375
Enercan M, Ozturk C, Kahraman S, Sarier M, Hamzaoglu A, Alanay A (2013) Osteotomies/spinal column resections in adult deformity. Eur Spine J 22(Suppl 2):S254–264
Oka S, Matsumiya H, Shinohara S, Kuwata T, Takenaka M, Chikaishi Y, Hirai A, Imanishi N, Kuroda K, Yamada S, Uramoto H, Nakamura E, Tanaka F (2016) Total or partial vertebrectomy for lung cancer invading the spine. Ann Med Surg (Lond) 12:1–4
Lin W, Xu H, Duan G, Xie J, Chen Y, Jiao B, Lan H (2018) Spine-shortening osteotomy for patients with tethered cord syndrome: a systematic review and meta-analysis. Neurol Res 40(5):340–363
Kelly MP, Lenke LG, Godzik J, Pellise F, Shaffrey CI, Smith JS, Lewis SJ, Ames CP, Carreon LY, Fehlings MG, Schwab F, Shimer AL (2017) Retrospective analysis underestimates neurological deficits in complex spinal deformity surgery: a Scoli-RISK-1 study. J Neurosurg Spine 27(1):68–73
Kawahara N, Tomita K, Kobayashi T, Abdel-Wanis ME, Murakami H, Akamaru T (2005) Influence of acute shortening on the spinal cord: an experimental study. Spine 30(6):613–620
Ji L, Dang XQ, Lan BS, Wang KZ, Huang YJ, Wen B, Duan HH, Ren F (2013) Study on the safe range of shortening of the spinal cord in canine models. Spinal Cord 51(2):134–138
Qiu F, Yang JC, Ma XY, Xu JJ, Yang QL, Zhou X, Xiao YS, Hu HS, Xia LH (2015) Relationship between spinal cord volume and spinal cord injury due to spinal shortening. PLoS ONE 10(5):e0127624
Modi HN, Suh SW, Hong JY, Yang JH (2011) The effects of spinal cord injury induced by shortening on motor evoked potentials and spinal cord blood flow: an experimental study in Swine. J Bone Joint Surg Am 93(19):1781–1789
Santillan A, Nacarino V, Greenberg E, Riina HA, Gobin YP, Patsalides A (2012) Vascular anatomy of the spinal cord. J Neurointerv Surg 4(1):67–74
Bican O, Minagar A, Pruitt AA (2013) The spinal cord: a review of functional neuroanatomy. Neurol Clin 31(1):1–18
Huang S, Xiang L, Huang Y, Wang F, Ji L (2018) Electrophysiological monitoring techniques for spinal cord function in a canine model. Int J Clin Exp Med 11(6):5986–5991
Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, Popovich PG (2017) Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J Neurosci Off J Soc Neurosci 37(13):3568–3587
Kobrine AI, Doyle TF, Martins AN (1975) Local spinal cord blood flow in experimental traumatic myelopathy. J Neurosurg 42(2):144–149
Cawthon DF, Senter HJ, Stewart WB (1980) Comparison of hydrogen clearance and 14C-antipyrine autoradiography in the measurement of spinal cord blood flow after severe impact injury. J Neurosurg 52(6):801–807
Martirosyan NL, Feuerstein JS, Theodore N, Cavalcanti DD, Spetzler RF, Preul MC (2011) Blood supply and vascular reactivity of the spinal cord under normal and pathological conditions. J Neurosurg Spine 15(3):238–251
Fujimaki Y, Kawahara N, Tomita K, Murakami H, Ueda Y (2006) How many ligations of bilateral segmental arteries cause ischemic spinal cord dysfunction? An experimental study using a dog model. Spine 31(21):E781–789
Ueda Y, Kawahara N, Tomita K, Kobayashi T, Murakami H, Nambu K (2005) Influence on spinal cord blood flow and function by interruption of bilateral segmental arteries at up to three levels: experimental study in dogs. Spine 30(20):2239–2243
Kato S, Kawahara N, Tomita K, Murakami H, Demura S, Fujimaki Y (2008) Effects on spinal cord blood flow and neurologic function secondary to interruption of bilateral segmental arteries which supply the artery of Adamkiewicz: an experimental study using a dog model. Spine 33(14):1533–1541
Mazensky D, Flesarova S, Sulla I (2017) Arterial blood supply to the spinal cord in animal models of spinal cord injury. A review. Anat Rec (Hoboken, NJ: 2007) 300(12):2091–2106
Kobayashi S, Yoshizawa H, Shimada S, Guerrero AR, Miyachi M (2013) Changes of blood flow, oxygen tension, action potential and vascular permeability induced by arterial ischemia or venous congestion on the spinal cord in canine model. J Orthop Res Off Publ Orthop Res Soc 31(1):139–146
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M (2011) The blood-spinal cord barrier: morphology and clinical implications. Ann Nneurol 70(2):194–206
Maikos JT, Shreiber DI (2007) Immediate damage to the blood-spinal cord barrier due to mechanical trauma. J Neurotrauma 24(3):492–507
Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, Zhou H, Ning G, Kong X, Feng S (2018) Microenvironment imbalance of spinal cord injury. Cell Transpl 27(6):853–866
Casha S, Zygun D, McGowan MD, Bains I, Yong VW, Hurlbert RJ (2012) Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain J Neurol 135(Pt 4):1224–1236
Kumar H, Ropper AE, Lee SH, Han I (2017) Propitious therapeutic modulators to prevent blood-spinal cord barrier disruption in spinal cord injury. Mol Neurobiol 54(5):3578–3590
Sharma HS (2011) Early microvascular reactions and blood-spinal cord barrier disruption are instrumental in pathophysiology of spinal cord injury and repair: novel therapeutic strategies including nanowired drug delivery to enhance neuroprotection. J Neural Transm 118(1):155–176
Sharma HS, Vannemreddy P, Patnaik P, Patnaik S, Mohanty S (2006) Histamine receptors influence blood-spinal cord barrier permeability, edema formation, and spinal cord blood flow following trauma to the rat spinal cord. Acta Neurochir Supplement 96:316–321
Loane DJ, Byrnes KR (2010) Role of microglia in neurotrauma. Neurother J Am Soc Exp Neurother 7(4):366–377
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L (1998) Acute inflammatory response in spinal cord following impact injury. Exp Neurol 151(1):77–88
Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ (2010) Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain A J Neurol 133(Pt 2):433
Anwar MA, Shehabi TSA, Eid AH (2016) Inflammogenesis of secondary spinal cord injury. Front Cell Neurosci 10(98):1–24
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12(7):388–399
Bains M, Hall ED (1822) Antioxidant therapies in traumatic brain and spinal cord injury. Biochem Biophys Acta 5:675–684
Hall ED, Wang JA, Bosken JM, Singh IN (2016) Lipid peroxidation in brain or spinal cord mitochondria after injury. J Bioenerg Biomembr 48(2):169–174
Ogura Y, Watanabe K, Hosogane N, Tsuji T, Ishii K, Nakamura M, Toyama Y, Chiba K, Matsumoto M (2011) Severe progressive scoliosis due to huge subcutaneous cavernous hemangioma: a case report. Scoliosis 6:3
Arlet V, Odent T, Aebi M (2003) Congenital scoliosis. Eur Spine J 12(5):456–463
Chu WC, Man GC, Lam WW, Yeung BH, Chau WW, Ng BK, Lam TP, Lee KM, Cheng JC (2008) Morphological and functional electrophysiological evidence of relative spinal cord tethering in adolescent idiopathic scoliosis. Spine 33(6):673–680
Dearolf WW, Betz RR, Vogel LC, Levin J, Clancy M, Steel HH (1990) Scoliosis in pediatric spinal cord-injured patients. J Pediatr Orthop 10(2):214–218
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 81371347) and Key Research and Development Plan of Shaanxi Province (No. 2018ZDXM-SF-057).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The animal models were performed by Le Ji, Wenchen Ji, Binshang Lan and Lisong Heng. SSEP monitoring was performed by Yajuan Huang and Min Feng. Material preparation, data collection and analysis were performed by Xiaoying Ma, Shengli Huang and Jingyuan Li. The first draft of the manuscript was written by Le Ji and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ji, L., Ma, X., Ji, W. et al. Safe range of shortening the middle thoracic spine, an experimental study in canine. Eur Spine J 29, 616–627 (2020). https://doi.org/10.1007/s00586-019-06268-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-06268-8